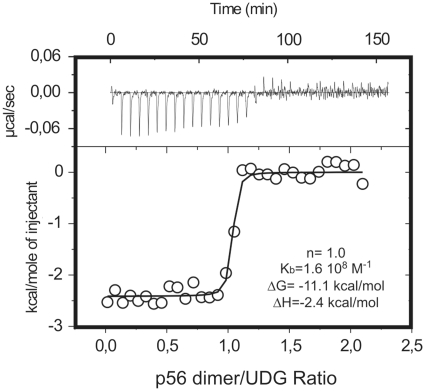
Figure 1.
Stoichiometry of the p56–UDG interaction as deduced from isothermal titration calorimetry (ITC). UDG solutions were titrated with p56 inhibitor at 25°C in 10 mM sodium phosphate, pH 6.5 and 100 mM NaCl. The experimental curves were fitted assuming a single set of sites on the enzyme. The obtanied stoichiometry factor (n), binding constant (Kb), binding free energy (ΔG) and enthalpy (ΔH) values are shown.