Figure 3.
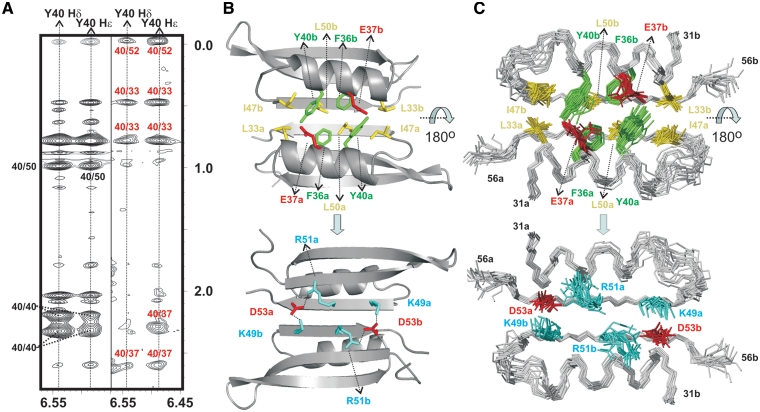
(A) Double-filtered (left) and half-filtered (right) NOESY experiments acquired for p56 at 35°C (buffer conditions: 100 mM NaCl and 10 mM sodium phosphate, pH 5.0). Inter-protein NOE contacts involving the aromatic ring of Y40 and residues E37, L33 and L52 are clearly observable in the half-filtered spectrum (labelled in red). (B) Structural details of p56 dimerization interface. Protein side-chains involved in relevant protein–protein contacts are highlighted (aromatic, in green; aliphatic, in yellow; positively charged, in cyan; negatively charged, in red). The protein backbone is shown in grey. Different monomeric units are labelled with subscripts a and b. Top and bottom views are related by a 180o rotation around the x-axis. (C) Ensemble of 20 NMR structures showing the inhibitor side-chains involved in dimerization.