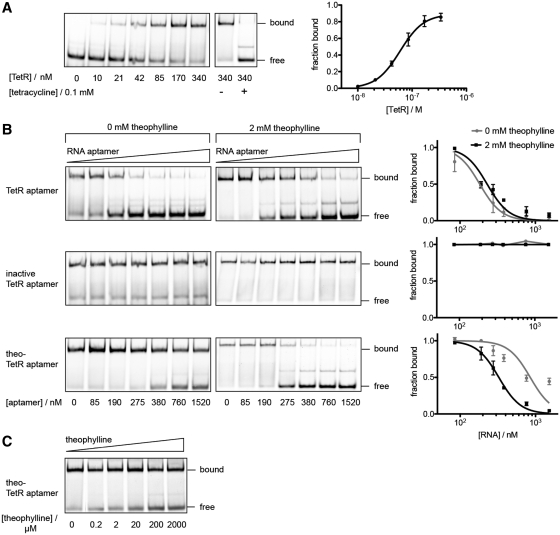
Figure 5.
In vitro competition assays of RNA aptamers withCy5-labeled dsDNA tetO for TetR binding. (A) Quantification of concentration-dependent binding of purified TetR protein to tetO resulted in an apparent Kd = 60 nM. TetR was released from DNA upon addition of tetracycline. (B) While the inactive TetR aptamer has no influence, the active variant competes with tetO for TetR binding independent of theophylline (Kc,no theo = 58 nM and Kc, 2 mM theo = 76 nM). Binding of the theophylline-responsive variant to TetR, however, is strongly improved in the presence of 2 mM theophylline (Kc,no theo = 276 nM and Kc, 2 mM theo = 106 nM). (C) Theophylline-dependent competition of theo-TetR intramer with tetO for TetR binding. If not stated separately, TetR concentration was kept constant at 340 nM, tetO at 190 nM and theo-TetR intramer at 275 nM.