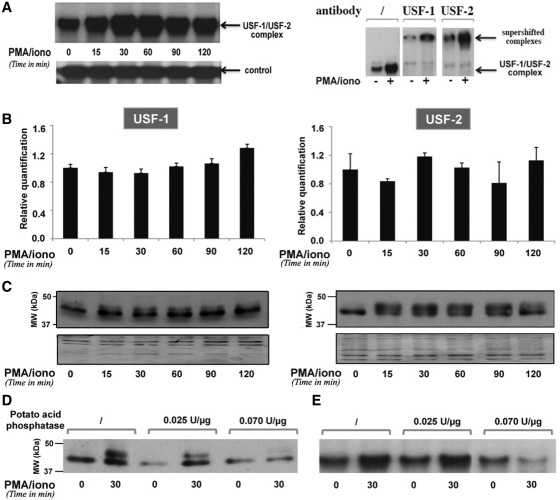
Figure 6.
PMA/ionomycin-induced phosphorylation of USF increases its DNA-binding affinity for the BLV E-box4 motif. (A) EMSAs were performed with nuclear extracts from YR2 cells mock treated or treated with PMA/ionomycin for different periods of time and a radiolabeled probe corresponding to the BLV E-box4 motif. Nuclear extracts from mock- or PMA/ionomycin-treated (for 30 min) YR2 cells were used in supershift experiments with the E-box4 radiolabeled probe alone or with an antibody directed against USF-1 or USF-2. A second not yet identified complex binding to the same probe was presented as a loading control. (B) USF-1 and USF-2 mRNA expression levels in mock- versus PMA/ionomycin-treated YR2 cells were measured by RT–qPCR. Results are presented as histograms indicating the PMA/ionomycin induction (n-fold) with respect to the mock-treated mRNA level, which was arbitrarily attributed a value of 1. Each value is the mean ± SEM of two independent experiments performed in triplicate. (C) Nuclear extracts from (A) were used in western blot experiments to detect USF-1 and USF-2 protein expression levels. As a loading control, the membrane was stained with Coomassie blue. (D) Nuclear extracts from mock- or PMA/ionomycin-treated YR2 cells were treated or not with increasing concentrations of potato acid phosphatase, resolved by SDS–PAGE and analyzed by immunoblotting with an antibody directed against USF-1. (E) Nuclear extracts from (D) were used in EMSAs experiments with the E-box4 radiolabeled probe.