Abstract
Three base pairs in the T-stem are primarily responsible for the sequence-specific interaction of tRNA with Escherichia coli and Thermus thermophilus EF-Tu. While the amino acids on the surface of EF-Tu that contact aminoacyl-tRNA (aa-tRNA) are highly conserved among bacteria, the T-stem sequences of individual tRNA are variable, making it unclear whether or not this protein–nucleic acid interaction is also sequence specific in other bacteria. We propose and validate a thermodynamic model that predicts the ΔG° of any tRNA to EF-Tu using the sequence of its three T-stem base pairs. Despite dramatic differences in T-stem sequences, the predicted ΔG° values for the majority of tRNA classes are similar in all bacteria and closely match the ΔG° values determined for E. coli tRNAs. Each individual tRNA class has evolved to have a characteristic ΔG° value to EF-Tu, but different T-stem sequences are used to achieve this ΔG° value in different bacteria. Thus, the compensatory relationship between the affinity of the tRNA body and the affinity of the esterified amino acid is universal among bacteria. Additionally, we predict and validate a small number of aa-tRNAs that bind more weakly to EF-Tu than expected and thus are candidates for acting as activated amino acid donors in processes outside of translation.
INTRODUCTION
The complex of elongation factor Tu (EF-Tu) and GTP binds aminoacyl-tRNA (aa-tRNA), and the resulting ternary complex binds ribosomes and participates in a multistep decoding pathway (1–3). While all 43 bacterial elongator aa-tRNAs appear to bind EF-Tu•GTP with similar affinities (4–7), studies with misacylated tRNAs indicate that this uniformity is the result of offsetting variable contributions of the esterified amino acid and tRNA body to the total binding affinity (8–10). The side chain of the amino acid fits into a large asymmetric pocket in EF-Tu, and the different amino acids contribute up to 2.8 kcal/mol to the total ΔG° of the ∼−10 kcal/mol that is observed for a typical aa-tRNA (9,11). Protein and tRNA mutagenesis experiments established that while many of the contacts between EF-Tu and tRNA contribute to ΔG°, the interaction of three protein residues with three adjacent base pairs in the T-stem is primarily responsible for the 3.6 kcal/mol range of ΔG° contributed by the different tRNA bodies (12–16). Subsequent experiments analyzing the kinetics of decoding of tRNAs engineered to have different ΔG° values established that tight-binding aa-tRNAs release from EF-Tu•GDP more slowly and thereby lower the rate of peptide bond formation (17). This suggests that the observed uniform ΔG° of all aa-tRNAs binding to EF-Tu is the result of an evolutionary optimization by two opposing selective pressures. The T-stem sequence of each tRNA evolved to be tight enough to initially bind to EF-Tu, but not too tight to limit its rate of release from EF-Tu•GDP during ribosomal decoding.
The above principles guiding the sequence specificity of the EF-Tu•aa-tRNA interaction were deduced from experiments performed using either Escherichia coli or Thermus thermophilus EF-Tu and mutations made in four different tRNA bodies. In this article, we explore whether these principles can be extended to all of the tRNAs in all bacterial species. Thus, do EF-Tu proteins from other bacteria recognize their corresponding tRNAs using the same sequence-specific interactions with the three T-stem base pairs? If this is largely true, exceptions could lead to refinements of the recognition model or to the identification of individual tRNAs that do not bind EF-Tu. If this is not true for all bacteria, it may be possible to deduce when the sequence-specific recognition rules emerged in bacterial evolution.
MATERIALS AND METHODS
Sequence analysis and ΔG° prediction
A total of 247 reviewed non-redundant (>90% identical) bacterial EF-Tu sequences were downloaded from Uniprot (http://www.uniprot.org) and aligned using clustal X (18). A total of 37 706 bacterial tRNA sequences from 629 bacterial species were downloaded from a genomic tRNA database http://gtrnadb.ucsc.edu/ (19). Separated tRNACAU classes were downloaded from (20) while tRNAUCA classes were separated using Tfam 1.3 http://tfam.lcb.uu.se/ (21). tRNAs in each anticodon class were subjected to a multiple sequence alignment using ClustalX. All sequences which were >94% identical were then removed using Jalview (22), yielding a total of 6113 non-redundant tRNAs. The major anticodon classes of tRNAs were then further analyzed to predict their ΔG° to EF-Tu.
ΔΔG° values for single base pair mutations in the T-stems of three E. coli tRNAs were calculated relative to the sequence of yeast tRNAPhe (15). For each tRNA position (49–65, 50–64 and 51–63), the single base pair ΔΔG°s were then averaged among the three E. coli tRNAs yielding 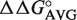 (Table 2). To calculate the affinity of each bacterial tRNA, the ΔG° of yeast Phe-tRNAPhe was added with
(Table 2). To calculate the affinity of each bacterial tRNA, the ΔG° of yeast Phe-tRNAPhe was added with 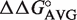 (49–65),
(49–65), 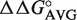 (50–64) and
(50–64) and 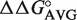 (51–63) based upon the sequence of each tRNA. The
(51–63) based upon the sequence of each tRNA. The 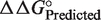 values were combined into 0.5 kcal/mol bins (23), and the number of tRNAs in each bin were fit to a Gaussian curve using Kaleidagraph (Synergy Software).
values were combined into 0.5 kcal/mol bins (23), and the number of tRNAs in each bin were fit to a Gaussian curve using Kaleidagraph (Synergy Software).
Table 2.
ΔΔG° values of single base pair mutations in three tRNAs
| tRNAPhe ΔΔG° a (kcal/mol) | tRNALeu ΔΔG° ab (kcal/mol) | tRNAThr ΔΔG° c (kcal/mol) |
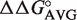 (kcal/mol) (kcal/mol) |
|
|---|---|---|---|---|
| 49–65 CG | 0.0 | 0.0 | 0.0 | 0.0 |
| 49–65 AU | −0.5 | −0.3 | −0.8 | −0.5 |
| 49–65 GC | −0.4 | −0.2 | −0.7 | −0.4 |
| 49–65 UA | −0.1 | N.D. | −0.2 | −0.2 |
| 49–65 GU | −0.8 | −0.9 | −1.1 | −0.9 |
| 50–64 UA | 0.0 | 0.0 | N.D. | 0.0 |
| 50–64 AU | −0.1 | 0.1 | N.D. | 0.0 |
| 50–64 GC | −0.2 | 0.3 | N.D. | 0.0 |
| 50–64 CG | −0.5 | 0.1 | N.D. | −0.2 |
| 50–64 GU | 1.3 | 1.4 | N.D. | 1.4 |
| 50–64 UG | 0.3 | 0.5 | N.D. | 0.4 |
| 51–63 GC | 0.0 | 0.0 | 0.0 | 0.0 |
| 51–63 AU | 0.8 | 1.4 | 1.0 | 1.1 |
| 51–63 CG | 0.5 | 0.6 | 0.4 | 0.5 |
| 51–63 UA | 0.9 | 1.0 | 1.2 | 1.0 |
| 51–63 GU | 1.0 | 1.1 | 0.2 | 0.8 |
| 51–63 UG | 1.0 | N.D. | N.D. | 1.0 |
| 51–63 AC | 0.2 | N.D. | N.D. | 0.2 |
Materials
Expression and purification of T. thermophilus EF-Tu, Yeast PheRS and E. coli PheRS were performed as described previously (9,12,15). tRNAs genes were chemically synthesized (IDT) in two fragments with at least 20 overlapping base pairs and extended using Taq DNA polymerase. tRNAs were transcribed by T7 RNA polymerase, gel purified and aminoacylated with [3H]-Phe as previously described (15).
EF-Tu-binding assay
The dissociation rate (koff) from EF-Tu•GTP was determined on ice in Buffer A (50 mM HEPES pH 7.0, 20 mM MgCl2, 0.5 M NH4Cl, 5 mM DTT, 20 µM GTP, 3 mM phosphoenolpyruvate and 50 µg/ml of pyruvate kinase) using a modified ribonuclease protection assay as described (15). KD was calculated using a previously determined kon of 1.1 × 105 M−1s−1 (15). ΔG° was calculated using ΔG° = −RT × Ln (KD). All measurements were performed in triplicate.
RESULTS
Sequence conservation in the interface between EF-Tu and aa-tRNA
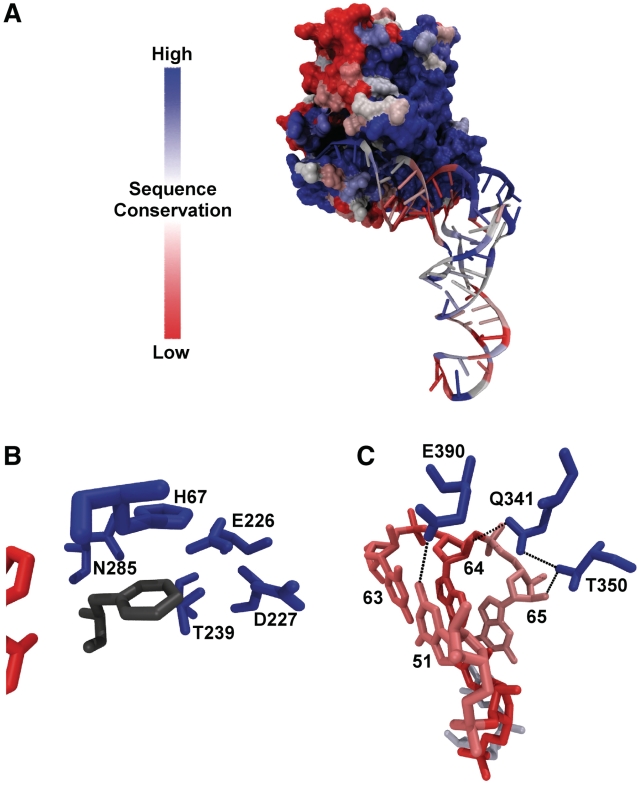
Mutagenesis of E. coli and T. thermophilus EF-Tu have identified 15 amino acids in the interface with aa-tRNA that contribute to the ΔG° of binding, including 3 in the amino acid-binding pocket and 12 in the region that binds the tRNA (3,12–14,24) (S. J. Chapman, E. Y. Yikilmaz personal communication). In cases where the same mutation has been made in both proteins, the effects are very similar. Alignment of 247 bacterial EF-Tu sequences indicate that 13 of these 15 thermodynamically important amino acids are universally (>99%) conserved and the remaining two are very (>80%) conserved but have substitutions of chemically similar amino acids in some species. When mapped on the structure of the ternary complex (Figure 1A), the extreme conservation of the part of the protein that contacts aa-tRNA is evident, including the binding pocket for the esterified amino acid (Figure 1B) and the sequence-specific recognition sites in the T-stem (Figure 1C). This high degree of conservation suggests that all bacterial EF-Tus may recognize aa-tRNAs in the same way.
Figure 1.
Sequence conservation in the interface between EF-Tu and aa-tRNA. Percent amino acid identity among bacterial EF-Tu sequences mapped onto the structure of yeast Phe-tRNAPhe bound to T. aquaticus EF-Tu (11). Sequence conservation on tRNA using a bits scale (56) is similarly mapped. (A) Global structure of the ternary complex. (B) The esterified amino acid binding pocket with esterified phenylalanine is shown in grey. (C) The T-stem recognition sites.
In contrast to EF-Tu, the sequences of the acceptor and T helices of tRNA which contact EF-Tu are highly variable among bacteria (Figure 1A). In particular, the three T-stem base pairs that are primarily responsible for sequence-specific binding are not very conserved (Figure 1C) (15). Using a genomic tRNA database (19) curated to remove duplicate sequences, 251 different combinations of the six residues were found in 6113 bacterial tRNAs. As summarized in Table 1, this variability in T-stem sequence is also present in each of the 45 individual tRNA classes defined by their anticodon sequence. At one extreme, the 167 bacterial 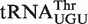 (
(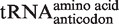 ) contain 53 different six-base sequences, and at the other, the 78
) contain 53 different six-base sequences, and at the other, the 78 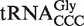 contain 10 different six-base sequences. This variability in T-stem sequences among bacterial tRNAs may mean that the rules for tRNA recognition are not universal among bacteria.
contain 10 different six-base sequences. This variability in T-stem sequences among bacterial tRNAs may mean that the rules for tRNA recognition are not universal among bacteria.
Table 1.
Predicted ΔG° values of each tRNA anticodon class
| tRNA anticodon class | Number of tRNAs | Number of 3-bp combinations | Measured ΔG° Phe-tRNAX (kcal/mol)d | Mean Calculated ΔG° Phe-tRNAX (kcal/mol) | σ | R |
|---|---|---|---|---|---|---|
| Ala CGC | 58 | 20 | −10.3 | 0.50 | 0.999 | |
| Ala GGC | 92 | 23 | −11.0 | −10.0 | 0.53 | 0.996 |
| Ala UGC | 93 | 25 | −10.0 | 0.52 | 0.998 | |
| Arg ACGa | 149 | 31 | −9.6 | −9.4 | 0.32 | 0.990 |
| Arg CCGa | 161 | 45 | −9.5 | 0.58 | 0.974 | |
| Arg CCU | 207 | 51 | −9.6 | 0.59 | 0.992 | |
| Arg UCU | 226 | 41 | −9.3 | 0.49 | 0.983 | |
| Arg UCGa | 66 | 23 | −9.4 | 0.32 | 0.987 | |
| Asn GUU | 160 | 23 | −9.4 | −9.4 | 0.60 | 0.998 |
| Asp GUC | 75 | 16 | −11.5 | −11.0 | 0.33 | 0.988 |
| Cys GCA | 175 | 30 | −10.1 | −9.9 | 0.62 | 0.992 |
| Gln CUG | 83 | 32 | −8.8 | −8.9 | 0.50 | 0.984 |
| Gln UUG | 88 | 32 | −9.1 | 0.56 | 0.997 | |
| Glu CUC | 70 | 17 | −10.3 | 0.50 | 0.998 | |
| Glu UUC | 117 | 21 | −12.2 | −10.4 | 0.48 | 0.997 |
| Gly CCC | 78 | 10 | −11.0 | 0.34 | 0.991 | |
| Gly GCC | 76 | 13 | −11.2 | −11.0 | 0.39 | 0.980 |
| Gly UCC | 99 | 19 | −10.8 | 0.68 | 0.981 | |
| His GUG | 180 | 22 | −10.7 | 0.56 | 0.997 | |
| Ile GAU | 93 | 34 | −9.2 | −9.4 | 0.36 | 0.998 |
| Leu CAA | 237 | 26 | −10.0 | 0.50 | 0.998 | |
| Leu CAG | 146 | 22 | −10.0 | −9.9 | 0.51 | 0.981 |
| Leu GAG | 152 | 23 | −10.4 | 0.60 | 0.989 | |
| Leu UAA | 232 | 26 | −10.3 | 0.43 | 1.000 | |
| Leu UAG | 194 | 20 | −10.2 | 0.49 | 0.941 | |
| Lys CUU | 117 | 30 | −9.4 | 0.86 | 0.989 | |
| Lys UUU | 151 | 35 | −10.0 | −9.7 | 0.76 | 0.980 |
| Met/fMet/Ile CAU | 320 | 69 | −9.5 | 0.94 | 0.970 | |
| Met CAUb | 89 | 34 | −9.9 | −9.9 | 0.85 | 0.926 |
| fMet CAUb | 43 | 24 | −8.9 | −9.1 | 0.55 | 0.973 |
| Ile CAUb | 101 | 31 | −9.3 | 0.48 | 0.984 | |
| Phe GAAa | 118 | 34 | −9.9 | −9.4 | 0.82 | 0.943 |
| Pro CGG | 83 | 20 | −9.8 | 0.36 | 0.993 | |
| Pro GGG | 86 | 24 | −9.8 | 0.56 | 0.995 | |
| Pro UGGa | 141 | 25 | −9.9 | −9.6 | 0.54 | 0.994 |
| Sec/Trp UCA | 63 | 36 | −8.2 | 1.2 | 0.706 | |
| Sec UCAc | 56 | 15 | −8.0 | −8.2 | 1.2 | 0.706 |
| Trp UCAc | 7 | 6 | −9.6 | 0.75 | 0.895 | |
| Ser CGA | 162 | 20 | −10.2 | 0.35 | 0.993 | |
| Ser GCU | 176 | 20 | −10.2 | 0.37 | 1.000 | |
| Ser GGA | 170 | 17 | −10.3 | 0.32 | 1.000 | |
| Ser UGAa | 218 | 20 | −9.6 | −10.1 | 0.32 | 0.923 |
| Thr CGU | 148 | 48 | −11.0 | −10.0 | 0.67 | 0.995 |
| Thr GGU | 158 | 41 | −10.1 | 0.52 | 0.999 | |
| Thr UGU | 167 | 53 | −9.8 | 0.57 | 0.982 | |
| Trp CCAa | 154 | 37 | −9.0 | −9.4 | 0.40 | 0.995 |
| Tyr GUA | 139 | 31 | −8.6 | −9.2 | 0.38 | 0.997 |
| Val CAC | 64 | 25 | −9.5 | 0.45 | 0.996 | |
| Val GAC | 137 | 33 | −9.7 | 0.41 | 0.998 | |
| Val UACa | 141 | 28 | −9.3 | −9.4 | 0.49 | 0.925 |
A thermodynamic model for predicting ΔG° for any tRNA
To avoid making and assaying all of the many different T-stem sequences in the database, we took advantage of a thermodynamic model to predict the affinity of each variant from a more limited set of data. Experiments measuring the binding affinities of single base pair mutations in the T-stems of three different tRNAs to either E. coli or T. thermophilus EF-Tu have been performed (15,17,25). Table 2 presents the ΔΔG° values for the different single base pair substitutions of the 49–65, 50–64 and 51–63 bp in the three tRNA backgrounds calculated using the sequence of yeast tRNAPhe as a reference. With the single exception of the G51–U63 pair in Thr-tRNAThr, the ΔΔG° values agree closely for the three data sets, despite the fact that different EF-Tu proteins were used. This argues that the sequence dependence of tRNA binding is very similar in different tRNA bodies. These ΔΔG° values for each individual tRNA mutation were averaged to yield 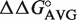 values which describe the change in free energy for any given single base pair mutation. Depending on the sequence, the KD of a tRNA can be affected by as much as 26-fold at each position.
values which describe the change in free energy for any given single base pair mutation. Depending on the sequence, the KD of a tRNA can be affected by as much as 26-fold at each position.
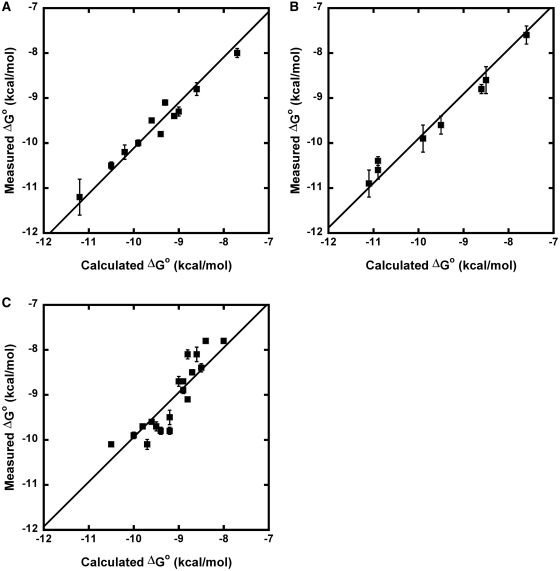
The 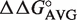 values derived from single base pair substitutions were usually able to accurately predict the ΔG° of multiple base pair substitutions. Figure 2 compares the measured ΔG° of multiple base pair substitutions in either Phe-tRNAPhe, Thr-tRNAThr, or Val-tRNAVal with the values of ΔG° calculated by adding the ΔG° of the wild-type tRNA to the appropriate
values derived from single base pair substitutions were usually able to accurately predict the ΔG° of multiple base pair substitutions. Figure 2 compares the measured ΔG° of multiple base pair substitutions in either Phe-tRNAPhe, Thr-tRNAThr, or Val-tRNAVal with the values of ΔG° calculated by adding the ΔG° of the wild-type tRNA to the appropriate 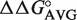 values from Table 2. In each case, the majority of the experimental ΔG° values fit the predicted values within experimental error, yielding lines with unitary slope. While there are several discrepancies between the experimental and predicted values, the vast majority are associated with tRNAThr where the very rare mismatched A52–C62 pair modifies the contribution of the adjacent 51–63 pair (25). In those cases where the A52–C62 pair in tRNAThr is changed to the more common G52–C62 pair, the ΔG° values of multiple base pair mutants are more accurately predicted. Thus, it appears that the thermodynamic contributions of the 3 bp are independent of one another in most cases, permitting the ΔG° values of T-stems containing multiple base pair substitutions to be estimated using the relatively few
values from Table 2. In each case, the majority of the experimental ΔG° values fit the predicted values within experimental error, yielding lines with unitary slope. While there are several discrepancies between the experimental and predicted values, the vast majority are associated with tRNAThr where the very rare mismatched A52–C62 pair modifies the contribution of the adjacent 51–63 pair (25). In those cases where the A52–C62 pair in tRNAThr is changed to the more common G52–C62 pair, the ΔG° values of multiple base pair mutants are more accurately predicted. Thus, it appears that the thermodynamic contributions of the 3 bp are independent of one another in most cases, permitting the ΔG° values of T-stems containing multiple base pair substitutions to be estimated using the relatively few 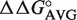 values.
values.
Figure 2.
Calculation of ΔG° of multiple base pair mutants. Comparison of experimental ΔG° values of multiple base pair mutants to ΔG° values calculated from 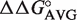 values. Calculated ΔG° = ΔG° (wild-type aa-tRNA) +
values. Calculated ΔG° = ΔG° (wild-type aa-tRNA) + 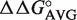 49–65 +
49–65 + 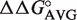 50–64 +
50–64 + 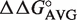 51–63 (A) yeast Phe-tRNAPhe to T. thermophilus EF-Tu (15), (B) E. coli
51–63 (A) yeast Phe-tRNAPhe to T. thermophilus EF-Tu (15), (B) E. coli  to E. coli EF-Tu (17) and (C)
to E. coli EF-Tu (17) and (C)  to E. coli EF-Tu (25).
to E. coli EF-Tu (25).
Calculating ΔG° for all bacterial tRNAs
Of the 6113 non-redundant bacterial tRNA sequences, 5849 (96%) have T-stem sequences for which single base pair 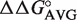 values are available for all 3 bp. For each bacterial tRNA, we chose to calculate their phenylalanylated versions using the ΔG° = −10.1 kcal/mol of Phe-tRNAPhe (15) and the appropriate
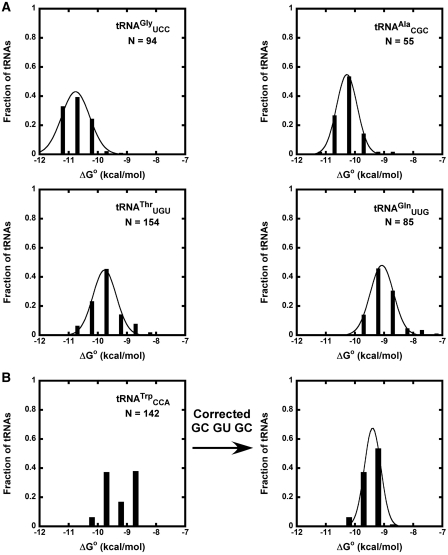
values are available for all 3 bp. For each bacterial tRNA, we chose to calculate their phenylalanylated versions using the ΔG° = −10.1 kcal/mol of Phe-tRNAPhe (15) and the appropriate 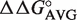 values associated with their T-stem sequence. By using a common esterified amino acid, facile comparison of the thermodynamic contributions of different tRNA bodies can be made both within a class and among classes. In addition, there is a considerable amount of experimental data for tRNAs misacylated with phenylalanine available for comparison (8,15). For each of the 45 different tRNA anticodon classes, calculated ΔG° values were grouped into bins of 0.5 kcal/mol, a bin size appropriate for the number of samples (23) and the error associated with the ΔΔG° values (8,15). Most of the resulting distributions of calculated ΔG° values fit well to a Gaussian distribution (Figure 3A), although several tRNA classes had ΔG° distributions with a significant second peak at a lower calculated ΔG° (Figure 3B). In many cases, the cause of this secondary peak can be attributed to the high preponderance of one six-base sequence. This particular sequence (G49C65, G50U64, G51C63) is one of the few that was previously found to be inaccurately calculated from the single base pair ΔΔG° values (Figure 2) (15). When the experimental ΔG° for this ‘non-additive’ T stem (15) was substituted for the calculated ΔG°, the fit of many tRNA classes was substantially improved (Figure 3B). The mean ΔG°, R and σ values describing the distributions of all tRNA classes made after this correction are summarized in Table 1.
values associated with their T-stem sequence. By using a common esterified amino acid, facile comparison of the thermodynamic contributions of different tRNA bodies can be made both within a class and among classes. In addition, there is a considerable amount of experimental data for tRNAs misacylated with phenylalanine available for comparison (8,15). For each of the 45 different tRNA anticodon classes, calculated ΔG° values were grouped into bins of 0.5 kcal/mol, a bin size appropriate for the number of samples (23) and the error associated with the ΔΔG° values (8,15). Most of the resulting distributions of calculated ΔG° values fit well to a Gaussian distribution (Figure 3A), although several tRNA classes had ΔG° distributions with a significant second peak at a lower calculated ΔG° (Figure 3B). In many cases, the cause of this secondary peak can be attributed to the high preponderance of one six-base sequence. This particular sequence (G49C65, G50U64, G51C63) is one of the few that was previously found to be inaccurately calculated from the single base pair ΔΔG° values (Figure 2) (15). When the experimental ΔG° for this ‘non-additive’ T stem (15) was substituted for the calculated ΔG°, the fit of many tRNA classes was substantially improved (Figure 3B). The mean ΔG°, R and σ values describing the distributions of all tRNA classes made after this correction are summarized in Table 1.
Figure 3.
Calculated ΔG° distributions for bacterial tRNAs. (A) Calculated ΔG° values for phenylalanylated 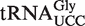 ,
, 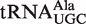 ,
, 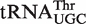 , and
, and 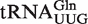 fit to Gaussian curves. Precisions of fit (R) and breadths (σ) of distributions are in Table 1. (B) Calculated ΔG° for
fit to Gaussian curves. Precisions of fit (R) and breadths (σ) of distributions are in Table 1. (B) Calculated ΔG° for 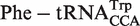 before and after tRNAs containing the non-additive T-stem (G49C65 G50U64 G51C63) were substituted with the measured value.
before and after tRNAs containing the non-additive T-stem (G49C65 G50U64 G51C63) were substituted with the measured value.
Nearly all bacterial tRNA anticodon classes have calculated ΔG° distributions that show an excellent fit to a Gaussian distribution (R > 0.9). The breadths of the distributions (σ) vary among the different tRNA classes but do not correlate with the number of T-stem sequences present in the class. The values of σ vary in a normal distribution between 0.3 and 0.8 kcal/mol, which is consistent with the error of the 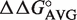 values used for the calculation. This argues that most bacteria show a similar characteristic ΔG° for each individual tRNA class. A closer examination of the T-stems of
values used for the calculation. This argues that most bacteria show a similar characteristic ΔG° for each individual tRNA class. A closer examination of the T-stems of 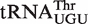 from different bacteria illustrates how the many different six residue sequences all calculate to a similar ΔG° value (Table 3). For example, while
from different bacteria illustrates how the many different six residue sequences all calculate to a similar ΔG° value (Table 3). For example, while 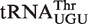 from Xanthomonas campestris uses the weak-binding C49–G65, tight-binding C50–G64, and intermediate-binding C51–G63 base pairs to achieve a calculated ΔG° = −9.8 kcal/mol, the
from Xanthomonas campestris uses the weak-binding C49–G65, tight-binding C50–G64, and intermediate-binding C51–G63 base pairs to achieve a calculated ΔG° = −9.8 kcal/mol, the 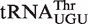 from Leuconostoc citreum uses a totally different combination of tight-binding G49–U65, intermediate-binding A50–U64 and weak-binding A51–U63 base pairs to give a nearly identical calculated ΔG° = −9.9 kcal/mol. Thus, although these two
from Leuconostoc citreum uses a totally different combination of tight-binding G49–U65, intermediate-binding A50–U64 and weak-binding A51–U63 base pairs to give a nearly identical calculated ΔG° = −9.9 kcal/mol. Thus, although these two 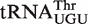 do not share any common nucleotides at the six positions, they have a similar calculated ΔG°. This strongly suggests that each T-stem evolved to reach its characteristic ΔG° and that all bacteria use similar rules for the sequence-specific recognition of tRNA by EF-Tu.
do not share any common nucleotides at the six positions, they have a similar calculated ΔG°. This strongly suggests that each T-stem evolved to reach its characteristic ΔG° and that all bacteria use similar rules for the sequence-specific recognition of tRNA by EF-Tu.
Table 3.
Calculated ΔG° values of bacterial 
| 49–65 | 50–64 | 51–63 | Calculated ΔG° (kcal/mol) | Number of tRNAs |
|---|---|---|---|---|
| GC | UA | CG | −10.0 | 19 |
| GC | CG | gu | −9.9 | 9 |
| GC | gu | GC | −9.1 | 9 |
| AU | GC | GC | −10.6 | 8 |
| GU | CG | gu | −10.4 | 7 |
| AU | UA | CG | −10.1 | 7 |
| GU | UA | au | −9.9 | 7 |
| GU | CG | au | −10.1 | 6 |
| GU | gu | GC | −9.6 | 6 |
| GC | CG | GC | −10.7 | 5 |
| GU | GC | gu | −10.2 | 5 |
| AU | CG | gu | −10.0 | 4 |
| GC | AU | GC | −10.5 | 3 |
| GC | CG | CG | −10.2 | 3 |
| GC | UA | ug | −9.5 | 3 |
| AU | CG | CG | −10.3 | 2 |
| GU | AU | au | −9.9 | 2 |
| AU | UA | gu | −9.8 | 2 |
| GC | UA | gu | −9.7 | 2 |
| cg | AU | CG | −9.6 | 2 |
| GC | UG | CG | −9.6 | 2 |
| AU | AU | au | −9.5 | 2 |
| cg | CG | gu | −9.5 | 2 |
| AU | gu | CG | −8.7 | 2 |
| cg | gu | CG | −8.2 | 2 |
| GC | GC | CG | −10.0 | 1 |
| cg | CG | CG | −9.8 | 1 |
| ua | UA | CG | −9.8 | 1 |
| GC | AU | gu | −9.7 | 1 |
| AU | UA | ug | −9.6 | 1 |
Calculated ΔG° values for 30 of the 53 most abundant T-stem sequences in bacterial 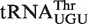 esterified with phenylalanine. Underlined tRNA base pairs bind tightly (GC), bold base pairs bind moderately (GC), and lowercase base pairs bind weakly (gc).
esterified with phenylalanine. Underlined tRNA base pairs bind tightly (GC), bold base pairs bind moderately (GC), and lowercase base pairs bind weakly (gc).
The mean ΔG° from the calculated distributions generally agree with the ΔG° values previously measured between T. thermophilus EF-Tu and E. coli tRNAs misacylated with phenylalanine (Table 1) (8). A clear exception is 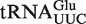 , which has a predicted mean ΔG° that is 1.8 kcal/mol weaker than the experimental ΔG° of the E. coli
, which has a predicted mean ΔG° that is 1.8 kcal/mol weaker than the experimental ΔG° of the E. coli 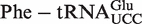 . While this may be a failure of our 3-bp model, it is also possible that the experimental value is incorrect since the unusually tight ΔG° of
. While this may be a failure of our 3-bp model, it is also possible that the experimental value is incorrect since the unusually tight ΔG° of 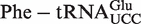 could only be estimated by extrapolation of data obtained at higher ionic strengths or temperatures (8).
could only be estimated by extrapolation of data obtained at higher ionic strengths or temperatures (8).
Different tRNA classes that are aminocylated by the same amino acid (termed isoacceptors) show very similar distributions centered about the same mean ΔG°. For example, similar mean ΔG° values were calculated for the three alanine isoacceptors: 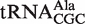 (−10.3 kcal/mol),
(−10.3 kcal/mol), 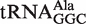 (−10.0 kcal/mol) and
(−10.0 kcal/mol) and 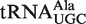 (−10.0 kcal/mol). Indeed, among all groups of isoacceptors, the mean calculated ΔG° values are generally within 0.3 kcal/mol, well within the error of the calculation. This high degree of similarity among the mean ΔG° values for isoacceptor tRNAs provides additional support to the model that the identity of the esterified amino acid largely drives the evolution of the T-stem sequence of the corresponding tRNA to have a common value of ΔG°.
(−10.0 kcal/mol). Indeed, among all groups of isoacceptors, the mean calculated ΔG° values are generally within 0.3 kcal/mol, well within the error of the calculation. This high degree of similarity among the mean ΔG° values for isoacceptor tRNAs provides additional support to the model that the identity of the esterified amino acid largely drives the evolution of the T-stem sequence of the corresponding tRNA to have a common value of ΔG°.
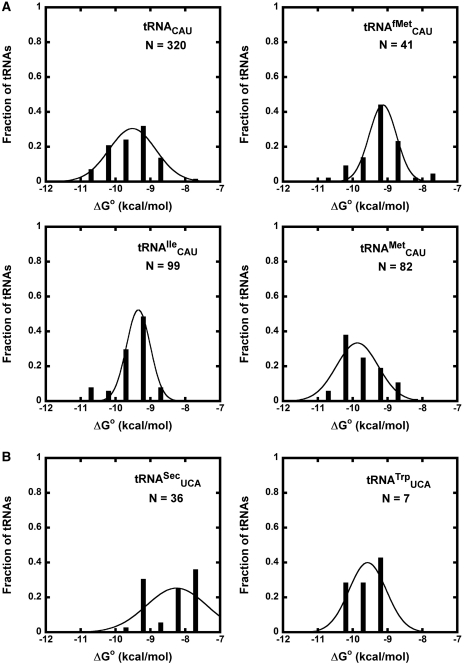
The four tRNA classes with the lowest R values and/or highest σ values were tRNACAU, tRNAUCA, 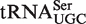 and
and 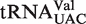 . For two of these, the poor fit of the distribution simply reflects the fact that two or more types of tRNA share the same anticodon. Thus, tRNACAU shows a very broad distribution of predicted ΔG° but contains both the initiator and elongator methionine tRNAs and
. For two of these, the poor fit of the distribution simply reflects the fact that two or more types of tRNA share the same anticodon. Thus, tRNACAU shows a very broad distribution of predicted ΔG° but contains both the initiator and elongator methionine tRNAs and 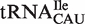 (20). However, if the tRNACAU are separated into subclasses using other sequence elements that are known to define them (20), the resulting calculated ΔG° distributions for the
(20). However, if the tRNACAU are separated into subclasses using other sequence elements that are known to define them (20), the resulting calculated ΔG° distributions for the 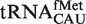 ,
, 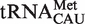 and
and 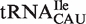 subclasses are all much narrower (Figure 4A). As expected, those tRNACAU identified as
subclasses are all much narrower (Figure 4A). As expected, those tRNACAU identified as 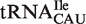 are predicted to bind similarly to the other isoacceptor,
are predicted to bind similarly to the other isoacceptor, 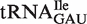 . Although the 41 tRNACAU identified as initiator methionine tRNAs were found to have T-stem sequences predicted to bind EF-Tu similar to
. Although the 41 tRNACAU identified as initiator methionine tRNAs were found to have T-stem sequences predicted to bind EF-Tu similar to 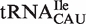 when both were phenylalanylated, the very weak binding of the esterified formyl methionine results in
when both were phenylalanylated, the very weak binding of the esterified formyl methionine results in 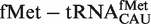 binding quite weakly. Since
binding quite weakly. Since 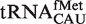 does not participate in elongation and is known to bind EF-Tu poorly (8,26,27), it is not surprising that it has evolved T-stem sequences that minimize association with EF-Tu (28). In addition to their weaker-binding T-stems, all but two of these
does not participate in elongation and is known to bind EF-Tu poorly (8,26,27), it is not surprising that it has evolved T-stem sequences that minimize association with EF-Tu (28). In addition to their weaker-binding T-stems, all but two of these 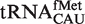 also contain a mismatched 1–72 bp which further destabilizes binding to EF-Tu (26). While the remaining tRNACAU species include the elongator methionine tRNAs predicted to bind EF-Tu quite well, the distribution of calculated ΔG° values remains broad with a large shoulder of more weakly binding tRNAs (Figure 4A). Some of these weaker binding
also contain a mismatched 1–72 bp which further destabilizes binding to EF-Tu (26). While the remaining tRNACAU species include the elongator methionine tRNAs predicted to bind EF-Tu quite well, the distribution of calculated ΔG° values remains broad with a large shoulder of more weakly binding tRNAs (Figure 4A). Some of these weaker binding 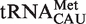 may actually be
may actually be 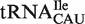 since distinguishing the two relies on the poorly conserved identity elements for TilS, the enzyme which modifies C34 (20,29).
since distinguishing the two relies on the poorly conserved identity elements for TilS, the enzyme which modifies C34 (20,29).
Figure 4.
Anticodon classes containing multiple tRNA types. (A) Calculated ΔG° values of Phe-tRNACAU sequences subdivided into 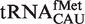 ,
, 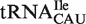 and
and 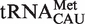 . (B) Calculated ΔG° values of Phe-tRNAUCA subdivided into
. (B) Calculated ΔG° values of Phe-tRNAUCA subdivided into 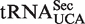 and
and 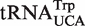 .
.
The broad calculated ΔG° distribution observed for the tRNAUCA sequence class (Figure 4B) is due to the presence of 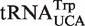 from Mycoplasmataceae (30,31) and
from Mycoplasmataceae (30,31) and 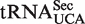 which both decode the UGA codon. Using TFAM to sort these tRNAs (21), the
which both decode the UGA codon. Using TFAM to sort these tRNAs (21), the 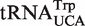 sequences give predicted ΔG° values similar to the more common
sequences give predicted ΔG° values similar to the more common 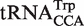 isoacceptor.
isoacceptor. 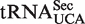 has an extended 8-bp acceptor stem that shifts its contact with EF-Tu by 1 bp such that the last base pair of the elongated acceptor-stem and the first two of the T-stem are recognized (32,33). By using these positions to calculate ΔG°, most
has an extended 8-bp acceptor stem that shifts its contact with EF-Tu by 1 bp such that the last base pair of the elongated acceptor-stem and the first two of the T-stem are recognized (32,33). By using these positions to calculate ΔG°, most 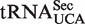 were predicted to bind weakly to EF-Tu, confirming previous experiments (32,33). Thus, similar to tRNACAU, the non-Gaussian distribution of ΔG° values observed among tRNAUCA sequences can probably be explained by the presence of multiple tRNA classes and thus does not conflict with the six-base model but rather supports it.
were predicted to bind weakly to EF-Tu, confirming previous experiments (32,33). Thus, similar to tRNACAU, the non-Gaussian distribution of ΔG° values observed among tRNAUCA sequences can probably be explained by the presence of multiple tRNA classes and thus does not conflict with the six-base model but rather supports it.
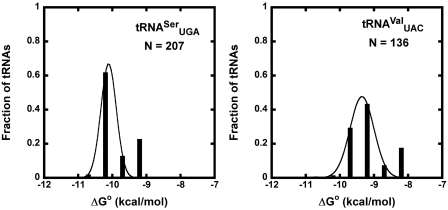
The two other tRNA classes, 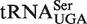 and
and 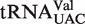 , each contain a small, distinct weaker affinity peak (Figure 5). This peak is unlikely to be explained by an unknown non-additive T-stem sequence since multiple T-stem sequences are present. It is possible that these two classes contain tRNAs with sequence elements outside of the three T-stem base pairs that strengthen binding to EF-Tu, but no obvious elements were discerned. As will be discussed below, another possible explanation is that the calculated ΔG° values are correct, but these classes contain many tRNA species that do not solely participate in translation elongation. Additional experiments measuring the EF-Tu-binding properties of the weaker binding members of these two classes will be needed to understand their anomalously predicted ΔG° values.
, each contain a small, distinct weaker affinity peak (Figure 5). This peak is unlikely to be explained by an unknown non-additive T-stem sequence since multiple T-stem sequences are present. It is possible that these two classes contain tRNAs with sequence elements outside of the three T-stem base pairs that strengthen binding to EF-Tu, but no obvious elements were discerned. As will be discussed below, another possible explanation is that the calculated ΔG° values are correct, but these classes contain many tRNA species that do not solely participate in translation elongation. Additional experiments measuring the EF-Tu-binding properties of the weaker binding members of these two classes will be needed to understand their anomalously predicted ΔG° values.
Figure 5.
Anticodon classes with poor fit to a Gaussian distribution. Calculated ΔG° values of  (R = 0.923) and
(R = 0.923) and  (R = 0.925).
(R = 0.925).
Individual tRNAs with abnormal ΔG° values
Of the 5849 calculated tRNA sequences, 25 have ΔG° values that are predicted to be tighter, and 19 have ΔG° values that are predicted to be weaker, than their corresponding mean ΔG° by >1.3 kcal/mol (Table 4). Given the large data set and the errors involved in the calculation, it is statistically unlikely that all of these tRNAs actually have anomalous ΔG° values. Nevertheless, this group of tRNAs, termed outliers, deserves further scrutiny. Indeed, one of the weaker outlier tRNAs is the 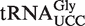 from Staphylococcus that functions as the glycine donor in the biosynthesis of pentapeptide crosslink in the cell wall (34–36). This tRNA was found to bind very poorly to Staphylococcus EF-Tu, suggesting it had evolved to avoid the translation machinery so that it could perform its specialized function (37). Those Staphylococcus species that contain this tRNA have a second copy of the
from Staphylococcus that functions as the glycine donor in the biosynthesis of pentapeptide crosslink in the cell wall (34–36). This tRNA was found to bind very poorly to Staphylococcus EF-Tu, suggesting it had evolved to avoid the translation machinery so that it could perform its specialized function (37). Those Staphylococcus species that contain this tRNA have a second copy of the 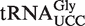 gene whose product binds EF-Tu more tightly and participates in translation. As expected, the predicted ΔG° value of this second tRNA is close to the mean of the
gene whose product binds EF-Tu more tightly and participates in translation. As expected, the predicted ΔG° value of this second tRNA is close to the mean of the 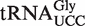 distribution. This successful identification of an aa-tRNA known to have a function outside of translation suggests that other outliers could have similar interesting functions. However, of the 44 outlier tRNAs, 41 possess T-stem sequences that have not been verified experimentally by inserting into tRNAPhe and therefore may have a ΔG° that was not predicted accurately by combining
distribution. This successful identification of an aa-tRNA known to have a function outside of translation suggests that other outliers could have similar interesting functions. However, of the 44 outlier tRNAs, 41 possess T-stem sequences that have not been verified experimentally by inserting into tRNAPhe and therefore may have a ΔG° that was not predicted accurately by combining 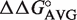 values. Alternatively, it is possible that some of these tRNAs resemble E. coli tRNAThr or tRNAfMet and possess sequence elements outside of the six residues in the T-stem that contribute to ΔG°. Finally, the predicted ΔG° values of the outlier tRNA may be accurate, indicating that the tRNA actually possesses an unusual ΔG° for binding EF-Tu in the organism.
values. Alternatively, it is possible that some of these tRNAs resemble E. coli tRNAThr or tRNAfMet and possess sequence elements outside of the six residues in the T-stem that contribute to ΔG°. Finally, the predicted ΔG° values of the outlier tRNA may be accurate, indicating that the tRNA actually possesses an unusual ΔG° for binding EF-Tu in the organism.
Table 4.
tRNAs with anomalous calculated ΔG° values
| tRNA | Species | Mean Isoacceptor ΔG° (kcal/mol) | Calculated ΔG° (kcal/mol) | tRNA Chimera ΔG° (kcal/mol) | tRNAPhe Chimera ΔG° (kcal/mol) |
|---|---|---|---|---|---|
| Another gene copy present | |||||
| Ala CGC | Sorangium cellulosum | −10.3 | −9.0 | −9.3 ± 0.6 | −8.9 ± 0.6 |
| Arg ACG | Sorangium cellulosum | −9.2 | −10.7 | ||
| Arg ACG | Pseudomonas aeruginosa | −9.2 | −10.7 | ||
| Asn GUU | Bradyrhizobium japonicum | −9.4 | −10.7 | ||
| Gln UUG | Mesorhizobium loti | −9.1 | −7.7 | ||
| Glu UUC | Thiomicrospira denitrificans ATCC 33890 | −10.4 | −8.1 | ||
| Glu UUC | Helicobacter pylori | −10.4 | −8.0 | −8.6 ± 0.04 | −8.4 ± 0.1 |
| Gly GCC | Acaryochloris marina MBIC11018 | −11.0 | −9.4 | ||
| Gly UCC | Staphylococcus species | −10.8 | −9.5 | −9.6 ± 0.3 | −9.7 ± 0.2 |
| Ile GAU | Mycobacterium vanbaalenii PYR-2 | −9.4 | −10.7 | ||
| Ile GAU | Leuconostoc mesenteroides ATCC 8294 | −9.4 | −8.1 | ||
| Thr CGU | Bifidobacterium longum | −10.0 | −8.1 | −8.3 ± 0.5 | −8.3 ± 0.3 |
| Only gene copy present | |||||
| Arg ACG | Aquifex aeolicus | −9.2 | −10.7 | ||
| Arg CCG | Geobacter sp FRC32 | −9.5 | −11.0 | ||
| Arg CCG | Candidatus Ruthia magnifica Cm Calyptogena magnifica | −9.5 | −11.0 | ||
| Arg CCG | Candidatus Vesicomyosocius okutanii HA | −9.5 | −11.0 | ||
| Arg CCG | Bifidobacterium longum | −9.5 | −11.0 | ||
| Arg CCU | Rubrobacter xylanophilus | −9.6 | −8.0 | −9.1 ± 0.4 | −8.4 ± 0.1 |
| Arg CCU | Fusobacterium nucleatum | −9.6 | −11.0 | −10.8 ± 0.6 | −10.9a |
| Arg UCU | Clavibacter michiganensis | −9.3 | −10.7 | ||
| Arg UCU | Lactobacillus sakei | −9.3 | −10.7 | ||
| Arg UCU | Zymomonas mobilis | −9.3 | −10.7 | ||
| Arg UCU | Acidiphilium cryptum JF-5 | −9.3 | −10.7 | ||
| Arg UCU | Chloroflexus aurantiacus J 10 fl | −9.3 | −10.7 | ||
| Arg UCU | Opitutus terrae PB90 | −9.3 | −10.7 | ||
| Arg UCU | Renibacterium salmoninarum ATCC 33209 | −9.3 | −10.7 | ||
| Arg UCU | Lactobacillus reuteri F275 | −9.3 | −10.7 | ||
| Arg UCU | Bacteroides fragilis NCTC 9434 | −9.3 | −10.6 | ||
| Arg UCU | Cytophaga hutchinsonii ATCC 33406 | −9.3 | −10.6 | ||
| Asn GUU | Borrelia afzelii Pko | −9.4 | −8.0 | ||
| Gln CUG | Lactobacillus acidophilus NCFM | −8.9 | −10.3 | ||
| Gln UUG | Solibacter usitatus Ellin6077 | −9.1 | −7.7 | ||
| Gln UUG | Candidatus Pelagibacter ubique HTCC1063 | −9.1 | −7.6 | ||
| Leu GAG | Bradyrhizobium japonicum | −10.4 | −9.1 | −9.8 ± 0.6 | −9.4a |
| Lys CUU | Ehrlichia ruminantium Gardel | −9.5 | −11.0 | ||
| Pro CGG | Lactobacillus acidophilus NCFM | −9.9 | −11.2 | ||
| Thr GGU | Ehrlichia canis Jake | −10.1 | −8.6 | ||
| Thr GGU | Mycoplasma genitalium | −10.1 | −8.2 | ||
| Thr GGU | Mycoplasma gallisepticum | −10.1 | −8.2 | ||
| Thr UGU | Corynebacterium urealyticum DSM 7110 | −9.8 | −11.2 | −10.5 ± 0.3 | −11.2a |
| Thr UGU | Mesorhizobium loti | −9.8 | −8.6 | −10.0 ± 0.7 | −8.7 ± 0.4 |
| Thr UGU | Candidatus Pelagibacter ubique HTCC1063 | −9.8 | −8.2 | ||
| Thr UGU | Dinoroseobacter shibae | −9.8 | −8.2 | ||
| Tyr GUA | Borrelia afzelii Pko | −9.2 | −10.5 | ||
Both calculated and measured ΔG° are for phenylalanylated tRNAs.
aΔG° measured in (15).
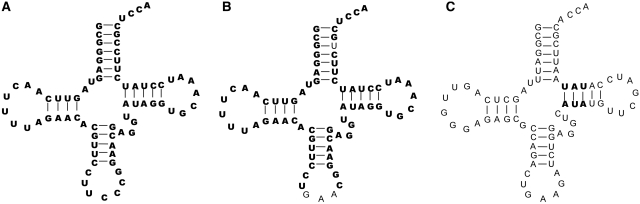
In order to test the above possibilities, 9 of the 44 outlier tRNAs were chosen and two chimeras were made for each (Figure 6). One chimera imported the six T-stem residues into tRNAPhe, thereby testing whether the 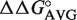 values were additive. The other chimera introduced the GAA anticodon and G3–U70 into the tRNA to permit aminoacylation with either Phe or Ala instead of the cognate amino acid. Since neither of these sequence changes affects EF-Tu affinity, this allows the outlier tRNA to be easily aminoacylated and its ΔG° to T. thermophilus EF-Tu to be measured (8,9). The weak-binding
values were additive. The other chimera introduced the GAA anticodon and G3–U70 into the tRNA to permit aminoacylation with either Phe or Ala instead of the cognate amino acid. Since neither of these sequence changes affects EF-Tu affinity, this allows the outlier tRNA to be easily aminoacylated and its ΔG° to T. thermophilus EF-Tu to be measured (8,9). The weak-binding 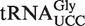 from Staphylococcus was included as a positive control. A second positive control was
from Staphylococcus was included as a positive control. A second positive control was 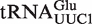 from Helicobacter pylori that also binds weakly to EF-Tu (38). Although the function of this tRNA is unknown, there is another copy of the tRNA gene (
from Helicobacter pylori that also binds weakly to EF-Tu (38). Although the function of this tRNA is unknown, there is another copy of the tRNA gene (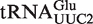 ) in Helicobacter that binds EF-Tu quite well. The experimental ΔG° values for the two chimeras of the nine outlier tRNAs are compared with their predicted values in Table 4. Both the
) in Helicobacter that binds EF-Tu quite well. The experimental ΔG° values for the two chimeras of the nine outlier tRNAs are compared with their predicted values in Table 4. Both the 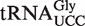 from Staphylococcus and the
from Staphylococcus and the 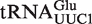 from Helicobacter pylori showed weak ΔG° values similar to the predicted values and to the values that had been previously determined (38). In addition, we show that a
from Helicobacter pylori showed weak ΔG° values similar to the predicted values and to the values that had been previously determined (38). In addition, we show that a 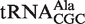 from Sorangium cellulosum and a
from Sorangium cellulosum and a 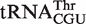 from Bifidobacterium longum that were predicted to bind weakly did indeed bind similarly to their predicted values. Since both of these organisms contain an additional copy of the tRNA gene with a T-stem sequence that is predicted to bind EF-Tu normally, these two tRNAs are also candidates to have specialized functions that do not involve translation. Eight other tRNAs that may have specialized functions are listed in Table 4.
from Bifidobacterium longum that were predicted to bind weakly did indeed bind similarly to their predicted values. Since both of these organisms contain an additional copy of the tRNA gene with a T-stem sequence that is predicted to bind EF-Tu normally, these two tRNAs are also candidates to have specialized functions that do not involve translation. Eight other tRNAs that may have specialized functions are listed in Table 4.
Figure 6.
Chimeras to experimentally test outlier tRNAs. (A) Sequence of Staphylococcus aureus 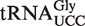 . (B) Chimera containing GAA anticodon and G3 U70 mutation to enable aminoacylation with either Phe or Ala. (C) Chimera with the S. aureus T-stem bases inserted into yeast tRNAPhe.
. (B) Chimera containing GAA anticodon and G3 U70 mutation to enable aminoacylation with either Phe or Ala. (C) Chimera with the S. aureus T-stem bases inserted into yeast tRNAPhe.
Thirty-two outlier tRNAs are the products of the only gene copy for the anticodon class present in the genome of the organism. This was unexpected because these tRNAs presumably are involved in decoding and thus should be subject to the same selective pressures on EF-Tu binding as all other tRNAs. For five of these tRNAs, the two chimeras were made and their ΔG° values determined (Table 4). Insertion of the T-stems of four of these variants into yeast tRNAPhe led to ΔG° close to the predicted values. However, the T-stem chimera from 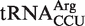 from Rubrobacter xylanophilus bound 0.4 kcal/mol more tightly than predicted, indicating that it is not an outlier but has a non-additive T stem. Consistent with this interpretation, the T-stem sequence from this tRNA (G49C65, G50U64, A51U63) is similar to the very abundant non-additive T stem discussed above. While the measured ΔG° values of the other four yeast tRNAPhe chimeras were close to the predicted values, the measured ΔG° values of the anticodon chimeras were quite different in three cases. Thus, Bradyrhizobium japonicum
from Rubrobacter xylanophilus bound 0.4 kcal/mol more tightly than predicted, indicating that it is not an outlier but has a non-additive T stem. Consistent with this interpretation, the T-stem sequence from this tRNA (G49C65, G50U64, A51U63) is similar to the very abundant non-additive T stem discussed above. While the measured ΔG° values of the other four yeast tRNAPhe chimeras were close to the predicted values, the measured ΔG° values of the anticodon chimeras were quite different in three cases. Thus, Bradyrhizobium japonicum 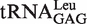 binds 0.7 kcal/mol tighter, Corynebacterium urealyticum
binds 0.7 kcal/mol tighter, Corynebacterium urealyticum 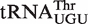 binds 0.7 kcal/mol weaker, and Mesorhizobium loti
binds 0.7 kcal/mol weaker, and Mesorhizobium loti 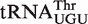 binds 1.4 kcal/mol tighter than the same six base sequences in tRNAPhe. In all three cases, these affinities are much closer to the mean ΔG° of their corresponding anticodon class, indicating that these tRNAs are also not outliers. Presumably, these three tRNAs have additional elements outside their T stem that affect the affinity to EF-Tu. Identification of these elements will require additional experiments, although candidate elements can be identified in the sequence. For example, the unexpectedly tight-binding
binds 1.4 kcal/mol tighter than the same six base sequences in tRNAPhe. In all three cases, these affinities are much closer to the mean ΔG° of their corresponding anticodon class, indicating that these tRNAs are also not outliers. Presumably, these three tRNAs have additional elements outside their T stem that affect the affinity to EF-Tu. Identification of these elements will require additional experiments, although candidate elements can be identified in the sequence. For example, the unexpectedly tight-binding 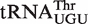 from Mesorhizobium loti contains a rare U5G68 U6G67 motif, which selectively binds a divalent metal ion in the major groove (39,40) and thereby could stabilize its interaction to EF-Tu. Finally, only one of the five selected outlier tRNAs from single copy genes actually has an anomalous ΔG° value. Both chimeras of the
from Mesorhizobium loti contains a rare U5G68 U6G67 motif, which selectively binds a divalent metal ion in the major groove (39,40) and thereby could stabilize its interaction to EF-Tu. Finally, only one of the five selected outlier tRNAs from single copy genes actually has an anomalous ΔG° value. Both chimeras of the 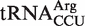 from Fusobacterium nucleatum bind significantly tighter to EF-Tu than
from Fusobacterium nucleatum bind significantly tighter to EF-Tu than 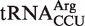 from other bacteria. While this tRNA is likely used in translation, it is unclear why the affinity has been selected to be so tight.
from other bacteria. While this tRNA is likely used in translation, it is unclear why the affinity has been selected to be so tight.
DISCUSSION
We developed a simple thermodynamic model to predict ΔG° of EF-Tu to different tRNA sequences. Our model assumes that the contribution of each of the three T-stem base pairs contributes independently to binding. This permits calculation of ΔG° for different T-stem sequences by summing the experimental 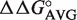 for the single base pair substitutions at the three sites. The model accurately predicts experimental ΔG° values for tRNAs containing multiple T-stem base pair changes irrespective of the tRNA body or EF-Tu used. When the model is used to predict ΔG° values for a large set of tRNAs from different bacteria, the tRNA anticodon classes have predicted ΔG° distributions which fit well to a Gaussian curve and have a width consistent with the experimental errors of the
for the single base pair substitutions at the three sites. The model accurately predicts experimental ΔG° values for tRNAs containing multiple T-stem base pair changes irrespective of the tRNA body or EF-Tu used. When the model is used to predict ΔG° values for a large set of tRNAs from different bacteria, the tRNA anticodon classes have predicted ΔG° distributions which fit well to a Gaussian curve and have a width consistent with the experimental errors of the 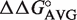 values. Their predicted mean ΔG° values are characteristic for each anticodon class despite the fact that their sequences vary considerably among bacteria. Since the variable affinity of tRNAs for EF-Tu is to compensate for the variable affinity contributed by the esterified amino acid, it appears that this evolutionary adaptation is maintained in all bacteria and uniform tRNA recognition rules are used.
values. Their predicted mean ΔG° values are characteristic for each anticodon class despite the fact that their sequences vary considerably among bacteria. Since the variable affinity of tRNAs for EF-Tu is to compensate for the variable affinity contributed by the esterified amino acid, it appears that this evolutionary adaptation is maintained in all bacteria and uniform tRNA recognition rules are used.
However, many tRNA classes showed a strong secondary peak in their distribution. The most common reason for this is that for a certain sequence (G49C65, G50U64, G51C63), the 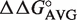 values at the three sites are not additive, so their ΔG° values were not accurately predicted by our simple model. Indeed, when experimental data was substituted for this common combination of six residues, all of the secondary peaks in the ΔG° distributions were substantially shifted into the main distribution. The presence of certain non-additive sequences is not surprising considering that the three ‘specificity’ base pairs are adjacent and that the protein contacts the 49–65 and 50–64 bp indirectly via the 2′ OH groups. Stacking between certain combinations of base pairs may form unusual helix geometries that alter binding. It is also possible that certain helical sequences selectively bind divalent ions that could influence ΔG° (39–41). Since only two such non-additive T-stem sequences were found out of 47 experimentally tested, it is unlikely that more than four additional ones will be found among the remaining 100 T stems that have not been measured.
values at the three sites are not additive, so their ΔG° values were not accurately predicted by our simple model. Indeed, when experimental data was substituted for this common combination of six residues, all of the secondary peaks in the ΔG° distributions were substantially shifted into the main distribution. The presence of certain non-additive sequences is not surprising considering that the three ‘specificity’ base pairs are adjacent and that the protein contacts the 49–65 and 50–64 bp indirectly via the 2′ OH groups. Stacking between certain combinations of base pairs may form unusual helix geometries that alter binding. It is also possible that certain helical sequences selectively bind divalent ions that could influence ΔG° (39–41). Since only two such non-additive T-stem sequences were found out of 47 experimentally tested, it is unlikely that more than four additional ones will be found among the remaining 100 T stems that have not been measured.
The poor fit of two distributions, tRNACAU and tRNAUCA, actually supports the six base model because the tRNA classes contain a mixture of two or three tRNA types with different EF-Tu-binding properties. When these two tRNA classes are sorted into their individual subclasses, the model is fairly effective at predicting characteristic ΔG° values. However, two anticodon classes, 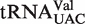 and
and 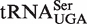 , have significantly poorer Gaussian fits due to the presence of a minor peak with weaker affinity. These minor peak tRNAs have several different six base sequences and do not appear in any particular set of organisms. It is possible that these tRNAs contain sequence elements outside the T stem that contribute to ΔG°, although no pattern of unusual sequences was discerned. A final possible explanation is that members of these particular anticodon classes also participate in non-translational functions that could place additional selective pressure on their sequence. For example, in addition to its function in translation, Ser-tRNASer often participates in the pentapeptide crosslink synthesis for peptidoglycan production (35,42,43) and has also been shown to participate in the biosynthesis of valanimycin (44). Perhaps for those tRNAs that have such dual roles, their T stems have evolved to be weaker for EF-Tu binding to allow a greater fraction of the molecules to be available for the non-translational biosynthetic enzymes (16,45,46). More experiments will be needed to determine whether the additional biosynthetic functions of aa-tRNAs negatively affect EF-Tu affinity.
, have significantly poorer Gaussian fits due to the presence of a minor peak with weaker affinity. These minor peak tRNAs have several different six base sequences and do not appear in any particular set of organisms. It is possible that these tRNAs contain sequence elements outside the T stem that contribute to ΔG°, although no pattern of unusual sequences was discerned. A final possible explanation is that members of these particular anticodon classes also participate in non-translational functions that could place additional selective pressure on their sequence. For example, in addition to its function in translation, Ser-tRNASer often participates in the pentapeptide crosslink synthesis for peptidoglycan production (35,42,43) and has also been shown to participate in the biosynthesis of valanimycin (44). Perhaps for those tRNAs that have such dual roles, their T stems have evolved to be weaker for EF-Tu binding to allow a greater fraction of the molecules to be available for the non-translational biosynthetic enzymes (16,45,46). More experiments will be needed to determine whether the additional biosynthetic functions of aa-tRNAs negatively affect EF-Tu affinity.
Our thermodynamic model also identified 44 rare ‘outlier’ tRNAs with predicted EF-Tu affinities that are either much tighter or weaker than expected and thus are candidates for unusual function. However, since many tRNAs contain T stems that had not been experimentally verified, it was important to prepare and measure the affinities of chimeras to confirm each putative outlier. Two examples of tRNAs that were known to bind EF-Tu poorly were successfully predicted: a 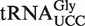 present in all Staphylococcus species and
present in all Staphylococcus species and 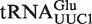 from Helicobacter pylori. We additionally verified that
from Helicobacter pylori. We additionally verified that 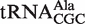 from Sorangium cellulosum and
from Sorangium cellulosum and 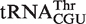 from Bifidobacterium longum bind EF-Tu poorly and, because these organisms have a second copy of the tRNA gene which is predicted to bind normally, these tRNAs are candidates to participate in a function that does not involve translation. At least four other such candidates of unusual function were found (Table 4).
from Bifidobacterium longum bind EF-Tu poorly and, because these organisms have a second copy of the tRNA gene which is predicted to bind normally, these tRNAs are candidates to participate in a function that does not involve translation. At least four other such candidates of unusual function were found (Table 4).
We also predicted many outlier tRNAs which were the only genomic copy of their respective anticodon class. When five chimeras from this group of tRNAs were tested, one contained a non-additive T-stem sequence and three contained elements outside the T stem which resulted in an affinity that was closer to the mean ΔG° for their respective anticodon classes. Therefore, four of the five predicted outlier tRNAs that were tested did not actually have anomalous ΔG° values and thus were not outliers. This suggests that many of the other predicted outliers from single copy genes in Table 4 do not actually have anomalous ΔG° values, supporting the view that the selective pressure to maintain the appropriate ΔG° value must be very strong. However, we found one predicted outlier from a single copy gene, 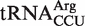 from Fusobacterium nucleatum, which binds to EF-Tu 1.2 kcal/mol more tightly than the mean ΔG° value for
from Fusobacterium nucleatum, which binds to EF-Tu 1.2 kcal/mol more tightly than the mean ΔG° value for 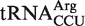 . It has been shown that aa-tRNAs with tighter than cognate affinities to EF-Tu release slowly into the ribosome after GTP hydrolysis (17), suggesting that this
. It has been shown that aa-tRNAs with tighter than cognate affinities to EF-Tu release slowly into the ribosome after GTP hydrolysis (17), suggesting that this 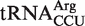 will cause slow translation of AGG codons and therefore may perform a unique regulatory function in Fusobacterium. It is possible that by slowing the translation rate of AGG codons, which compose only 0.23% of codons in Fusobacterium ORFs,
will cause slow translation of AGG codons and therefore may perform a unique regulatory function in Fusobacterium. It is possible that by slowing the translation rate of AGG codons, which compose only 0.23% of codons in Fusobacterium ORFs, 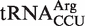 could be used to modulate translational efficiency of mRNAs enriched in AGG codons or it could be used to induce specific pauses to facilitate cotranslational protein folding (47,48). Another possible explanation for the tight affinity of this tRNA is that under conditions where the EF-Tu•GTP concentration is low, such as encountered during starvation, this tRNAArg would still function efficiently, possibly to translate AGG codons in starvation-induced genes. Indeed, it is known that this arginine isoacceptor is selectively charged upon starvation in E. coli (49). Future experiments will be needed uncover the role of this tight-binding tRNA.
could be used to modulate translational efficiency of mRNAs enriched in AGG codons or it could be used to induce specific pauses to facilitate cotranslational protein folding (47,48). Another possible explanation for the tight affinity of this tRNA is that under conditions where the EF-Tu•GTP concentration is low, such as encountered during starvation, this tRNAArg would still function efficiently, possibly to translate AGG codons in starvation-induced genes. Indeed, it is known that this arginine isoacceptor is selectively charged upon starvation in E. coli (49). Future experiments will be needed uncover the role of this tight-binding tRNA.
We have provided evidence that the mechanism for specific recognition of aa-tRNAs by EF-Tu is conserved in all bacteria. Indeed, the ΔG° values of individual tRNA classes are remarkably uniform among bacteria, especially considering the broad range of growth conditions encountered between organisms. Because the entire aa-tRNA interface of EF-Tu is almost universally conserved, bacterial tRNAs use the same three T-stem base pairs employed by E. coli to subtly modulate the affinities to EF-Tu in a way that compensates for the variable affinities of the esterified amino acid. This universality of the recognition rules was difficult to anticipate because of the extreme variability of bacterial T-stem sequences. Because two of the three specificity contacts involved interactions with the backbone of the RNA with unpredictable sequence specificity, the rules had to be determined empirically. As a result of quite variable contributions to ΔG° found at the three sites, multiple different T-stem sequences can give similar ΔG° values. The sequence-specific recognition of helices may be used by other RNA-binding proteins. For example, the many contacts between the helical regions of tRNA and aa-tRNA synthetases could contribute to sequence-specific binding as some experiments have suggested (50).
While all bacteria use the same mechanism of recognition of aa-tRNAs by EF-Tu, an analysis of protein and tRNA sequences indicates that eukaryotic and archaeal EF-1α recognize aa-tRNAs in a different manner. While no ternary complex structures with EF-1α are available, a GTP-bound form of an archaeal protein (51) permits an accurate structure-based sequence alignment with bacterial EF-Tu sequences. Although some of the residues that make up the amino acid-binding pocket in bacterial EF-Tu (H67, E226, D227, T239 and N285) are present in archaea (N285, D227), others are quite different from bacteria (Q226, V239 and F67). These changes are likely to alter the specificity of the pocket for different esterified amino acids (24,52). Although two of the three archaeal EF-1α residues (S350, D390 and V341) expected to interact with the T stem (13) are fairly similar to bacteria (T350 and E390), the third (Q341) is not. It is therefore likely that the combination of the three changes would be sufficient to alter the subtle hydrogen-bonding pattern used to achieve sequence- specific tRNA binding. In addition, if the bacterial 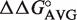 values are used to predict the ΔG° of archaeal tRNAs, it is clear that archaeal tRNAs often fit poorly to the corresponding bacterial distributions. For example, while bacterial tRNATrp binds rather weakly with a mean ΔG° = −9.4 kcal/mol (Table 1), applying our model yields a distribution of archaeal tRNATrp with two completely distinct peaks: one at −10.2 kcal/mol which is significantly tighter and the other at −8.7 kcal/mol which is significantly weaker. This suggests the way that archaeal EF-1α interacts with aa-tRNAs differs substantially from bacteria. Although no appropriate structure is available to permit alignment, it is likely that eukaryotic EF-1α also recognizes aa-tRNAs differently from bacteria. The differences in archaea and eukaryotic EF-1α are not entirely surprising as their translation systems contain many distinct features from bacteria, especially in initiation (53). While relatively little biochemical data is available, eukaryotic EF-1α also appears to require the presence of the esterified amino acid and GTP to bind tRNA (54,55). However, it is clear that additional quantitative data will be needed to understand how different archaeal or eukayotic tRNAs interact with EF-1α.
values are used to predict the ΔG° of archaeal tRNAs, it is clear that archaeal tRNAs often fit poorly to the corresponding bacterial distributions. For example, while bacterial tRNATrp binds rather weakly with a mean ΔG° = −9.4 kcal/mol (Table 1), applying our model yields a distribution of archaeal tRNATrp with two completely distinct peaks: one at −10.2 kcal/mol which is significantly tighter and the other at −8.7 kcal/mol which is significantly weaker. This suggests the way that archaeal EF-1α interacts with aa-tRNAs differs substantially from bacteria. Although no appropriate structure is available to permit alignment, it is likely that eukaryotic EF-1α also recognizes aa-tRNAs differently from bacteria. The differences in archaea and eukaryotic EF-1α are not entirely surprising as their translation systems contain many distinct features from bacteria, especially in initiation (53). While relatively little biochemical data is available, eukaryotic EF-1α also appears to require the presence of the esterified amino acid and GTP to bind tRNA (54,55). However, it is clear that additional quantitative data will be needed to understand how different archaeal or eukayotic tRNAs interact with EF-1α.
FUNDING
National Institutes of Health (Grant number GM037552 to O.C.U.). Funding for open access charge: GM037552.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors would like to thank Margaret Saks and Jonathan Widom for insightful discussions.
REFERENCES
- 1.Krab IM, Parmeggiani A. EF-Tu, a GTPase odyssey. Biochim. Biophys. Acta. 1998;1443:1–22. doi: 10.1016/s0167-4781(98)00169-9. [DOI] [PubMed] [Google Scholar]
- 2.Pape T, Wintermeyer W, Rodnina MV. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J. 1998;17:7490–7497. doi: 10.1093/emboj/17.24.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrader JM, Saks ME, Uhlenbeck OC. Ribosomes: Structure, Function, and Dynamics. New York: Springer Wien; 2010. [Google Scholar]
- 4.Louie A, Jurnak F. Kinetic studies of Escherichia coli elongation factor Tu-guanosine 5′-triphosphate-aminoacyl-tRNA complexes. Biochemistry. 1985;24:6433–6439. doi: 10.1021/bi00344a019. [DOI] [PubMed] [Google Scholar]
- 5.Louie A, Ribeiro NS, Reid BR, Jurnak F. Relative affinities of all Escherichia coli aminoacyl-tRNAs for elongation factor Tu-GTP. J. Biol. Chem. 1984;259:5010–5016. [PubMed] [Google Scholar]
- 6.Ott G, Schiesswohl M, Kiesewetter S, Forster C, Arnold L, Erdmann VA, Sprinzl M. Ternary complexes of Escherichia coli aminoacyl-tRNAs with the elongation factor Tu and GTP: thermodynamic and structural studies. Biochim. Biophys. Acta. 1990;1050:222–225. doi: 10.1016/0167-4781(90)90170-7. [DOI] [PubMed] [Google Scholar]
- 7.Asahara H, Uhlenbeck OC. Predicting the binding affinities of misacylated tRNAs for Thermus thermophilus EF-Tu.GTP. Biochemistry. 2005;44:11254–11261. doi: 10.1021/bi050204y. [DOI] [PubMed] [Google Scholar]
- 8.Asahara H, Uhlenbeck OC. The tRNA specificity of Thermus thermophilus EF-Tu. Proc. Natl Acad. Sci. USA. 2002;99:3499–3504. doi: 10.1073/pnas.052028599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dale T, Sanderson LE, Uhlenbeck OC. The affinity of elongation factor Tu for an aminoacyl-tRNA is modulated by the esterified amino acid. Biochemistry. 2004;43:6159–6166. doi: 10.1021/bi036290o. [DOI] [PubMed] [Google Scholar]
- 10.LaRiviere FJ, Wolfson AD, Uhlenbeck OC. Uniform binding of aminoacyl-tRNAs to elongation factor Tu by thermodynamic compensation. Science. 2001;294:165–168. doi: 10.1126/science.1064242. [DOI] [PubMed] [Google Scholar]
- 11.Nissen P, Kjeldgaard M, Thirup S, Polekhina G, Reshetnikova L, Clark BF, Nyborg J. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science. 1995;270:1464–1472. doi: 10.1126/science.270.5241.1464. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson LE, Uhlenbeck OC. Directed mutagenesis identifies amino acid residues involved in elongation factor Tu binding to yeast Phe-tRNA(Phe) J. Mol. Biol. 2007;368:119–130. doi: 10.1016/j.jmb.2007.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanderson LE, Uhlenbeck OC. Exploring the specificity of bacterial elongation factor Tu for different tRNAs. Biochemistry. 2007;46:6194–6200. doi: 10.1021/bi602548v. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson LE, Uhlenbeck OC. The 51-63 base pair of tRNA confers specificity for binding by EF-Tu. RNA. 2007;13:835–840. doi: 10.1261/rna.485307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrader JM, Chapman SJ, Uhlenbeck OC. Understanding the sequence specificity of tRNA binding to elongation factor Tu using tRNA mutagenesis. J. Mol. Biol. 2009;386:1255–1264. doi: 10.1016/j.jmb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy H, Ibba M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc. Natl Acad. Sci. USA. 2008;105:4667–4672. doi: 10.1073/pnas.0800006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrader JM, Chapman SJ, Uhlenbeck OC. Tuning the affinity of aminoacyl-tRNA to elongation factor Tu for optimal decoding. Proc. Natl Acad. Sci. USA. 2011;108:5215–5220. doi: 10.1073/pnas.1102128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan PP, Lowe TM. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 2009;37:D93–D97. doi: 10.1093/nar/gkn787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva FJ, Belda E, Talens SE. Differential annotation of tRNA genes with anticodon CAT in bacterial genomes. Nucleic Acids Res. 2006;34:6015–6022. doi: 10.1093/nar/gkl739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taquist H, Cui Y, Ardell DH. TFAM 1.0: an online tRNA function classifier. Nucleic Acids Res. 2007;35:W350–W353. doi: 10.1093/nar/gkm393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturges HA. The choice of a cass interval. J. Am. Stat. Assoc. 1926;21:65–66. [Google Scholar]
- 24.Roy H, Becker HD, Mazauric MH, Kern D. Structural elements defining elongation factor Tu mediated suppression of codon ambiguity. Nucleic Acids Res. 2007;35:3420–3430. doi: 10.1093/nar/gkm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saks ME, Sanderson LE, Choi DS, Crosby CM, Uhlenbeck OC. Functional consequences of T-stem mutations in E. coli tRNAThrUGU in vitro and in vivo. RNA. 2011;17:1038–1047. doi: 10.1261/rna.2427311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seong BL, RajBhandary UL. Mutants of Escherichia coli formylmethionine tRNA: a single base change enables initiator tRNA to act as an elongator in vitro. Proc. Natl Acad. Sci. USA. 1987;84:8859–8863. doi: 10.1073/pnas.84.24.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer W, Doi T, Ikehara M, Ohtsuka E, Sprinzl M. Interaction of methionine-specific tRNAs from Escherichia coli with immobilized elongation factor Tu. FEBS Lett. 1985;192:151–154. doi: 10.1016/0014-5793(85)80062-4. [DOI] [PubMed] [Google Scholar]
- 28.Stortchevoi A, Varshney U, RajBhandary UL. Common location of determinants in initiator transfer RNAs for initiator-elongator discrimination in bacteria and in eukaryotes. J. Biol. Chem. 2003;278:17672–17679. doi: 10.1074/jbc.M212890200. [DOI] [PubMed] [Google Scholar]
- 29.Ikeuchi Y, Soma A, Ote T, Kato J, Sekine Y, Suzuki T. molecular mechanism of lysidine synthesis that determines tRNA identity and codon recognition. Mol. Cell. 2005;19:235–246. doi: 10.1016/j.molcel.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Simoneau P, Li CM, Loechel S, Wenzel R, Herrmann R, Hu PC. Codon reading scheme in Mycoplasma pneumoniae revealed by the analysis of the complete set of tRNA genes. Nucleic Acids Res. 1993;21:4967–4974. doi: 10.1093/nar/21.21.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inamine JM, Ho KC, Loechel S, Hu PC. Evidence that UGA is read as a tryptophan codon rather than as a stop codon by Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum. J. Bacteriol. 1990;172:504–506. doi: 10.1128/jb.172.1.504-506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudinger J, Hillenbrandt R, Sprinzl M, Giege R. Antideterminants present in minihelix(Sec) hinder its recognition by prokaryotic elongation factor Tu. EMBO J. 1996;15:650–657. [PMC free article] [PubMed] [Google Scholar]
- 33.Baron C, Bock A. The length of the aminoacyl-acceptor stem of the selenocysteine-specific tRNA(Sec) of Escherichia coli is the determinant for binding to elongation factors SELB or Tu. J. Biol. Chem. 1991;266:20375–20379. [PubMed] [Google Scholar]
- 34.Bumsted RM, Dahl JL, Soll D, Strominger JL. Biosynthesis of the peptidoglycan of bacterial cell walls. X. Further study of the glycyl transfer ribonucleic acids active in peptidoglycan synthesis in Staphylococcus aureus. J. Biol. Chem. 1968;243:779–782. [PubMed] [Google Scholar]
- 35.Petit JF, Strominger JL, Soll D. Biosynthesis of the peptidoglycan of bacterial cell walls. VII. Incorporation of serine and glycine into interpeptide bridges in Staphylococcus epidermidis. J. Biol. Chem. 1968;243:757–767. [PubMed] [Google Scholar]
- 36.Roberts RJ. Structures of two glycyl-tRNAs from Staphylococcus epidermidis. Nat. New Biol. 1972;237:44–45. doi: 10.1038/newbio237044a0. [DOI] [PubMed] [Google Scholar]
- 37.Giannouli S, Kyritsis A, Malissovas N, Becker HD, Stathopoulos C. On the role of an unusual tRNAGly isoacceptor in Staphylococcus aureus. Biochimie. 2009;91:344–351. doi: 10.1016/j.biochi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Cathopoulis TJ, Chuawong P, Hendrickson TL. Conserved discrimination against misacylated tRNAs by two mesophilic elongation factor Tu orthologs. Biochemistry. 2008;47:7610–7616. doi: 10.1021/bi800369q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varani G, McClain WH. The G x U wobble base pair. A fundamental building block of RNA structure crucial to RNA function in diverse biological systems. EMBO Rep. 2000;1:18–23. doi: 10.1093/embo-reports/kvd001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ott G, Arnold L, Limmer S. Proton NMR studies of manganese ion binding to tRNA-derived acceptor arm duplexes. Nucleic Acids Res. 1993;21:5859–5864. doi: 10.1093/nar/21.25.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keel AY, Rambo RP, Batey RT, Kieft JS. A general strategy to solve the phase problem in RNA crystallography. Structure. 2007;15:761–772. doi: 10.1016/j.str.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villet R, Fonvielle M, Busca P, Chemama M, Maillard AP, Hugonnet JE, Dubost L, Marie A, Josseaume N, Mesnage S, et al. Idiosyncratic features in tRNAs participating in bacterial cell wall synthesis. Nucleic Acids Res. 2007;35:6870–6883. doi: 10.1093/nar/gkm778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lloyd AJ, Gilbey AM, Blewett AM, De Pascale G, El Zoeiby A, Levesque RC, Catherwood AC, Tomasz A, Bugg TD, Roper DI, et al. Characterization of tRNA-dependent peptide bond formation by MurM in the synthesis of Streptococcus pneumoniae peptidoglycan. J. Biol. Chem. 2008;283:6402–6417. doi: 10.1074/jbc.M708105200. [DOI] [PubMed] [Google Scholar]
- 44.Garg RP, Gonzalez JM, Parry RJ. Biochemical characterization of VlmL, a Seryl-tRNA synthetase encoded by the valanimycin biosynthetic gene cluster. J. Biol. Chem. 2006;281:26785–26791. doi: 10.1074/jbc.M603675200. [DOI] [PubMed] [Google Scholar]
- 45.Francklyn CS, Minajigi A. tRNA as an active chemical scaffold for diverse chemical transformations. FEBS Lett. 2010;584:366–375. doi: 10.1016/j.febslet.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.RajBhandary UL, Soll D. Aminoacyl-tRNAs, the bacterial cell envelope, and antibiotics. Proc. Natl Acad. Sci. USA. 2008;105:5285–5286. doi: 10.1073/pnas.0801193105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang G, Hubalewska M, Ignatova Z. Transient ribosomal attenuation coordinates protein synthesis and co-translational folding. Nat. Struct. Mol. Biol. 2009;16:274–280. doi: 10.1038/nsmb.1554. [DOI] [PubMed] [Google Scholar]
- 48.Plotkin JB, Kudla G. Synonymous but not the same: the causes and consequences of codon bias. Nat. Rev. Genet. 2011;12:32–42. doi: 10.1038/nrg2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dittmar KA, Sorensen MA, Elf J, Ehrenberg M, Pan T. Selective charging of tRNA isoacceptors induced by amino-acid starvation. EMBO Rep. 2005;6:151–157. doi: 10.1038/sj.embor.7400341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perona JJ, Hou YM. Indirect readout of tRNA for aminoacylation. Biochemistry. 2007;46:10419–10432. doi: 10.1021/bi7014647. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi K, Kikuno I, Kuroha K, Saito K, Ito K, Ishitani R, Inada T, Nureki O. Structural basis for mRNA surveillance by archaeal Pelota and GTP-bound EF1alpha complex. Proc. Natl Acad. Sci. USA. 2010;107:17575–17579. doi: 10.1073/pnas.1009598107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato A, Watanabe Y, Suzuki T, Komiyama M, Watanabe K, Ohtsuki T. Identification of the residues involved in the unique serine specificity of Caenorhabditis elegans mitochondrial EF-Tu2. Biochemistry. 2006;45:10920–10927. doi: 10.1021/bi060536i. [DOI] [PubMed] [Google Scholar]
- 53.Benelli D, Londei P. Translation initiation in Archaea: conserved and domain-specific features. Biochem. Soc. Trans. 2011;39:89–93. doi: 10.1042/BST0390089. [DOI] [PubMed] [Google Scholar]
- 54.Dreher TW, Uhlenbeck OC, Browning KS. Quantitative assessment of EF-1alpha.GTP binding to aminoacyl-tRNAs, aminoacyl-viral RNA, and tRNA shows close correspondence to the RNA binding properties of EF-Tu. J. Biol. Chem. 1999;274:666–672. doi: 10.1074/jbc.274.2.666. [DOI] [PubMed] [Google Scholar]
- 55.Gromadski KB, Wieden HJ, Rodnina MV. Kinetic mechanism of elongation factor Ts-catalyzed nucleotide exchange in elongation factor Tu. Biochemistry. 2002;41:162–169. doi: 10.1021/bi015712w. [DOI] [PubMed] [Google Scholar]
- 56.Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]