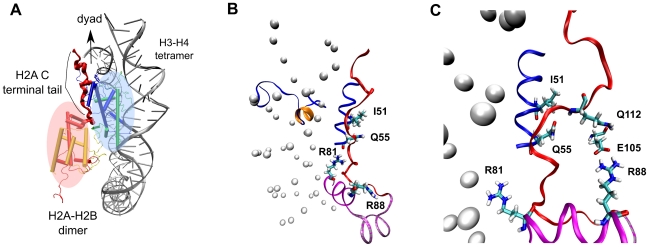
Figure 14. Regulation of nucleosome stability through H2A docking domain contacts.
(A) Positions of the H2A–H2B dimer and H3–H4 tetramer in a nucleosome illustrating that the interaction surface between the H2A–H2B dimer and H3–H4 tetramer is provided by the H2A C terminal tail. Histone dimers H3, H4, H2A and H2B are colored in blue, green, red and yellow, respectively. Part of the nucleosome structure is shown in cartoon representation for clarity of vision. (B)–(C) Disruption of contacts between the H3 tail  -helix (colored orange) and the DNA triggers change of interaction of histone arginines (Arg81 and Arg88). Newly formed polar contacts between Arg88, Glu105 and Gln112 destabilizes interaction of the H2A docking domain with closely lying amino acids (Ile51 and Gln55). Histone protein domains are shown as ribbons and DNA phosphorous atoms are shown as spheres. Histone domains are color coded as follows : H3
-helix (colored orange) and the DNA triggers change of interaction of histone arginines (Arg81 and Arg88). Newly formed polar contacts between Arg88, Glu105 and Gln112 destabilizes interaction of the H2A docking domain with closely lying amino acids (Ile51 and Gln55). Histone protein domains are shown as ribbons and DNA phosphorous atoms are shown as spheres. Histone domains are color coded as follows : H3 N (blue), H2A
N (blue), H2A 3 (magenta), H2A
3 (magenta), H2A C (grey), H2A docking domain (red).
C (grey), H2A docking domain (red).