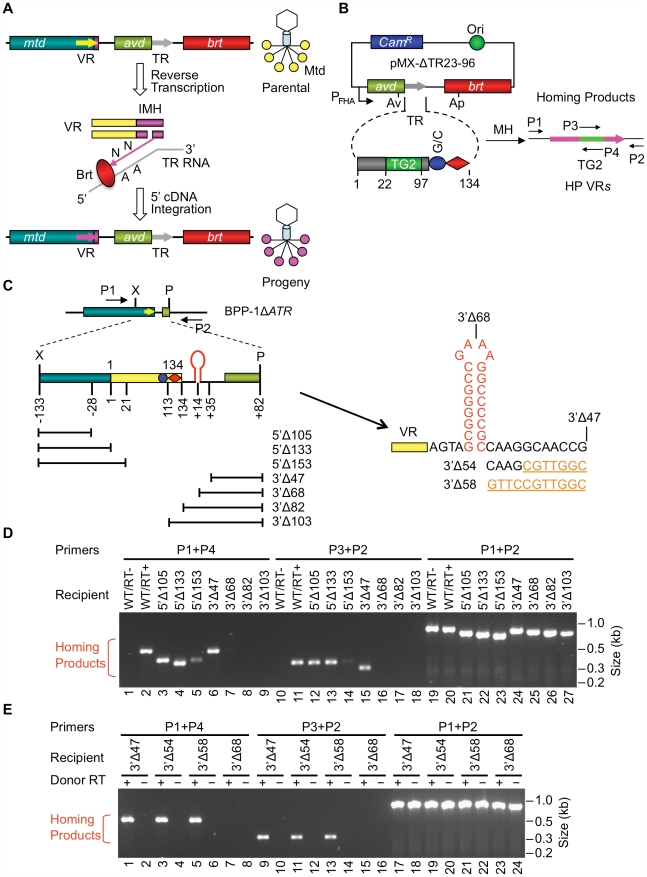
Figure 1. Boundaries of the BPP-1 DGR target sequence.
(A) Tropism switching by Bordetella phage BPP-1 is mediated by its DGR through mutagenic homing, which is proposed to occur through a target DNA-primed reverse transcription (TPRT) mechanism [4]. mtd, avd, brt, the variable and template repeats (VR and TR) are indicated. VR diversification leads to altered Mtd trimers at the distal tail fibers of progeny phages. (B) PCR-based DGR homing assay. Plasmid pMX-ΔTR23–96 carries the BPP-1 avd-TR-brt region placed downstream of the BvgAS-regulated Pfha promoter and contains a 30 bp insert (TG2) between TR positions 22 and 97 [4]. Grey and pink arrows represent TR and VR, respectively. Small horizontal arrows indicate primers used for homing assays: P1 and P2 are sense- and antisense-strand primers annealing upstream and downstream of VR, respectively; P3 and P4, sense- and antisense-strand primers, respectively, that anneal to TG2. CamR, chloramphenicol resistance gene. (C) BPP-1 DGR target region and deletion constructs. 5′ or 3′ deletions start from position −133 upstream of VR or position +82 downstream of VR, respectively. Lines below the target represent regions deleted. The region downstream of VR contains two 8 bp inverted repeats, which can potentially form a hairpin/cruciform structure. Constructs 3′Δ54 and 3′Δ58 were derived from 3′Δ47 by changing 7 and 11 residues downstream of the hairpin/cruciform structure to their complementary residues, respectively, and were assayed in E. (D) PCR-based DGR homing assays showed that BPP-1 target recognition does not require any sequence upstream of VR, but does require up to 35 bp downstream of VR, which includes the potential hairpin/cruciform-forming region. Donor RT+indicates pMX-ΔTR23–96, - indicates pMX-ΔTR23–96 with the SMAA mutation. (E) Fine mapping of the 3′ boundary of the BPP-1 DGR target with additional deletion constructs 3′Δ54 and 3′Δ58 showed that no sequences downstream of position +24 are required for target recognition.