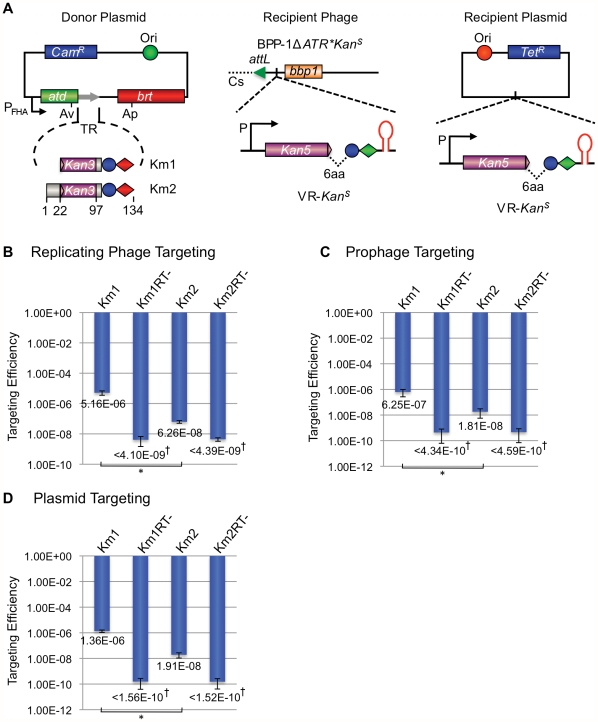
Figure 9. The BPP-1 DGR can be engineered to target a kanamycin-resistance gene.
(A) Donor and recipient constructs used for KanR gene targeting. The left panel shows two donor plasmids, pMX-Km1 and pMX-Km2, both with engineered TRs containing the last 36 bp of the KanR ORF. The KanR reporter gene on recipient VRs has a truncation of the last 6 codons and is placed upstream of the IMH and hairpin/cruciform elements. The recipient cassette was inserted between attL and bbp1 of phage BPP-1ΔATR* (Recipient Phage, middle) or in a pMMB208-derived plasmid (Recipient Plasmid, left). (B) Engineered BPP-1 DGRs can target a KanR reporter gene on a replicating phage. Targeting assays were carried out by phage BPP-1ΔATR*KanS lytic infection of RB50 cells carrying the indicated donor plasmids. Targeting efficiencies were determined as the relative numbers of KanR cells in lysogens generated with fresh RB50 cells and the progeny phages. The bars for Km1 and Km2 represent mean ± s.d. *P<0.01 in a Student's t test. (C) Engineered BPP-1 DGRs can target a KanR reporter gene on a prophage in the bacterial chromosome. BPP-1ΔATR*KanS lysogens transformed with indicated donor plasmids were used for targeting assays. Resulting cells were directly plated on selective (+Kan) or non-selective plates to determine relative numbers of KanR cells. The bars represent mean ± s.d. *P<0.05 in a Student's t test. (D) Engineered BPP-1 DGRs can target a KanR reporter gene on a plasmid. Following induction for donor plasmid expression and KanR gene targeting, RB50 cells transformed with both donor and recipient plasmids were plated on selective (+Kan) or non-selective plates to determine targeting efficiencies as in (C). The bars represent mean ± s.d. *P<0.002 in a Student's t test. †No KanR colonies were observed for RT-deficient donors and the numbers represent limits of detection.