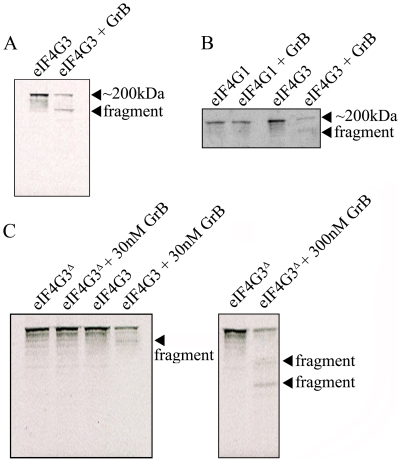
Figure 2. GrB-mediated cleave of eIF4G3 occurs at position D1408.
(A) Autoradiography of eIF4G3 treated with 30 nM GrB for 30 min. Treatment of eIF4G3 (∼200 kDa) with 30 nM GrB generates a proteolytic fragment; (n = 3 of 3 independent experiments). (B) The eIF4G3 isoform, eIF4G1, is not degraded by GrB. Loaded protein levels for eIF4G1 and eIF4G3 lanes are identical to their corresponding GrB treatment lanes; (n = 3 of 3 independent experiments). To visualize eIF4G3 fragments, eIF4G3 lanes were loaded at a higher concentration. (C) Putative P1 residue D1408 of eIF4G3 was mutagenized to alanine to generate the mutant eIF4G3Δ. eIF4G3 and eIF4G3Δ were synthesized in vitro and labeled with MetS35. These two proteins were treated with 30 and 300 nM GrB. Detectable degradation products are indicated with an arrow; (n = 3 of 3 independent experiments).