Abstract
Myotilin cDNA has been cloned for the first time from chicken muscles and sequenced. Ectopically expressed chicken and human YFP-myotilin fusion proteins localized in avian muscle cells in the Z-bodies of premyofibrils and the Z-bands of mature myofibrils. Fluorescence Recovery After Photobleaching (FRAP) experiments demonstrated that chicken and human myotilin were equally dynamic with 100% mobile fraction in premyofibrils and Z-bands of mature myofibrils. Seven myotilin mutants cDNAs (S55F, S55I, T57I, S60C, S60F, S95I, R405K) with known muscular dystrophy association localized in mature myofibrils in the same way as normal myotilin without affecting the formation and maintenance of myofibrils. N- and C-terminal halves of human myotilin were cloned and expressed as YFP fusions in myotubes and cardiomyocytes. N-terminal myotilin (aa 1–250) localized weakly in Z-bands with a high level of unincorporated protein and no adverse effect on myofibril structure. C-terminal myotilin (aa 251–498) localized in Z-bands and in aggregates. Formation of aggregated C-terminal myotilin was accompanied by the loss of Z-band localization of C-terminal myotilin and partial or complete loss of alpha-actinin from the Z-bands. In regions of myotubes with high concentrations of myotilin aggregates there were no alpha-actinin positive Z-bands or organized F-actin. The dynamics of the C-terminal-myotilin and N-terminal myotilin fragments differed significantly from each other and from full-length myotilin. In contrast, no significant changes in dynamics were detected after expression in myotubes of myotilin mutants with single amino acid changes known to be associated with myopathies.
Keywords: Myofibrillogenesis, sarcomere, premyofibril, mature myofibril, Z-band, FRAP, dynamics
INTRODUCTION
Myotilin, a 57-kDa protein, is one of the many components in the network of interacting proteins that form the Z-bands in vertebrate striated muscle [Salmikangas et al., 1999]. Deciphering the molecular interactions of Z-band proteins with multiple partners in the Z-band complex is a current challenge. A clear understanding of these interactions is important because it will help unravel the mechanism by which missense mutations in myotilin may cause muscular diseases such as Limb-Girdle Muscular Dystrophy in humans [Moreira et al., 2000; Shalaby et al., 2009; Selcen, 2011]. In common with other proteins in the Z-band complex, myotilin has multiple binding partners. These include alpha-actinin [Salmikangas et al., 1999], filamin [van derVen et al. 2000], actin [Salmikangas et al. 2003], and FATZ 1 and FATZ 2 [Gontier et al., 2005]. The known binding region of myotilin is in the C-terminal half of the molecule where two Ig-like domains are important for the antiparallel arrangement of myotilin dimers [Salmikangas et al., 2003] in addition to the reported protein binding.
In this study, we have cloned and sequenced the myotilin cDNA from the cardiac and skeletal muscles of chickens. We have also ectopically expressed EYFP-myotilin fusion proteins in cultured embryonic chicken cardiac and quail skeletal muscle cells by transfecting with pEYFP-Chi-Myotilin. The ectopically expressed fusion protein is localized in Z-bands in both types of muscle cells. In addition, we have determined the dynamics of wild type and mutated human myotilin molecules in muscle cells using FRAP (Fluorescence Recovery After Photobleaching) analysis. We also determined the dynamics of each of the two fragments of human myotilin designated by N-myotilin (1–250 amino acid residues) and C-myotilin (251- 498 amino acid residues; myotilin’s two Ig domains are in this sequence of amino acids) by using FRAP in cardiac and skeletal muscle cells isolated from avian embryos. The relative recovery of full-length myotilin after photobleaching is faster than the C-myotilin but slower than that of N-myotilin. Over-expression of C-myotilin, but not N-myotilin, led to the loss of myofibrils in both types of muscle cells. These results indicate the important role of the two Ig-domains for the maintenance of the Z-bands and myofibrils.
MATERIALS AND METHODS
Cloning and sequencing of myotilin from RNA isolated from embryonic and adult chicken muscle
RNA was isolated from embryonic and adult chicken hearts and skeletal muscles separately. cDNA was made from the total RNA (four samples separately) using oligo dT as the primer following published protocols [Zajdel et al., 2003; Wang et al., 2007, 2008]. Chicken myotilin cDNA was amplified by RT-P C R with the primer-pairs: Forward primer (P1) 5′-GGATGTTTAACTACGAACGT-3′; Reverse primer (P2) 5′-TTAAAGTTCATCGCTTTCA-3′ derived from NCBI Reference Sequence: XM_414618.2 for the predicted chicken myotilin cDNA sequence. The PCR amplified DNA was run in an agarose gel and the band was gel purified and cloned into a TA-cloning vector (Invitrogen). Nucleotide sequences of myotilin clones from adult heart and skeletal muscles were found to be identical suggesting the myotilin transcript expression in heart and skeletal muscle is from the same gene.
Expression constructs
Human myotilin cDNA was originally amplified by RT-PCR from human skeletal muscle mRNA and cloned into pEYFP (Clontech) as reported earlier [Wang et al. 2005]. Its N-terminus (1–250 aa residues), designated as Myotilin-N, and C-terminus (251–498 aa residues), denoted as Myotilin-C, fragments were cloned into pEYFP using previously reported methods. Chicken myotilin cDNA was amplified by RT-PCR as described above and subsequently cloned into pEYFP. Cerulean Fluorescent Protein (CeFP)-fused alpha-actinin was co-transfected into cells to label the Z-band of myofibrils [Wang et al., 2005; Sanger et al., 2009]. Site-directed mutagenesis of human myotilin cDNA at seven disease-associated sites (S55F, S55I, T57I, S60C, S60F, S95I, R405K) [Selcen, 2011] was carried out using Stratagene’s QuikChange site-directed mutagenesis kit and confirmed by DNA sequencing.
Cell Culture, Transfection, and Immunostaining
Embryonic quail myoblasts were isolated from 9-day-old quail embryos and plated on collagen-coated 35-mm MatTek (Ashland, MA) dishes at concentrations of 105 cells per dish according to procedures described in Dabiri et al. [1999]. Cardiomyocytes were isolated from the hearts of 10-day-old chick embryos as previously described [Dabiri et al., 1999], and plated at 3 × 105 cells per dish. The cardiac and skeletal muscle cells were transfected after 2 days of culture using Fugene HD transfection reagent. The transfection medium was replaced after two days with fresh muscle medium. Immunostaining of transfected cells was performed after 3 days of transfection as previously described [Dabiri et al., 1999]. The cells were fixed with buffered 3% paraformaldehyde, permeabilized, and stained with rhodamine-phalloidin.
Confocal imaging and Fluorescence Recovery After Photobleaching (FRAP)
Fluorescence images were acquired on either a Zeiss LSM 510 or a Leica SP5 confocal microscope. FRAP experiments were conducted using time points separated by 30-second intervals over 10 to 20 minutes collection period. The FRAP results were normalized and fitted to one or two exponential processes as previously reported [Wang et al. 2005; Sanger et al., 2010].
RESULTS
Cloning and sequencing of chicken myotilin from chicken heart and skeletal muscle
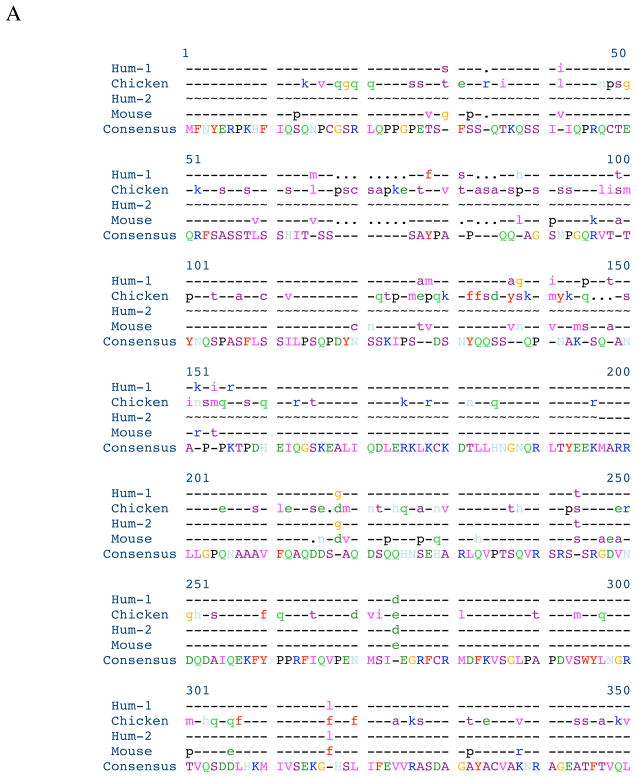
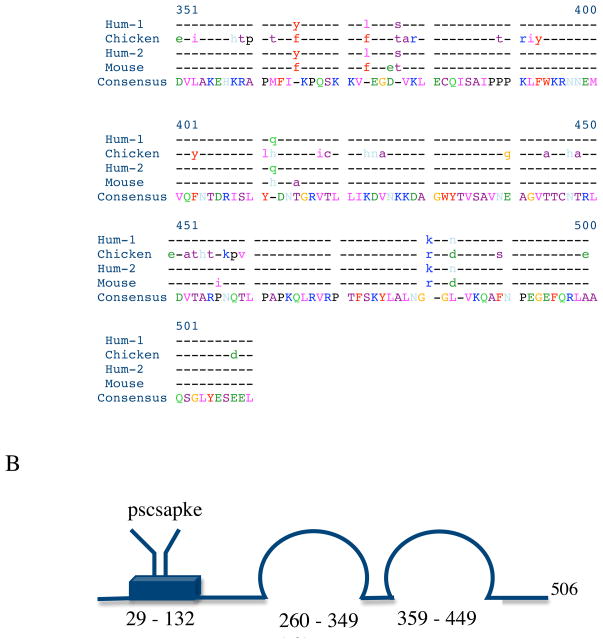
The strategy for cloning chicken myotilin was carried out by RT-PCR with the primer-pairs as described in the method section. The nucleotide sequence of the isolated plasmid DNA was determined to be identical with that of the predicted chicken myotilin sequence. The deduced sequence of the 506 amino acids of the chicken myotilin is shown aligned with the sequences of two alternatively spliced human variants and the mouse myotilin sequence in Figure 1A. Human variant 1 encodes 498 amino acid residues (GenBank number BC005376.1), eight fewer than in chicken. whereas transcript variant 2 (low molecular weight myotilin) encodes 314 amino acid residues (NCBI Reference Sequence: NM_001135940.1. Interestingly, an octapeptide “Pro.Ser.Cystein.Ser.Ala.Pro.Lys.Glu” present at the beginning of the serine-rich region (resides 29 – 124) of the chicken myotilin (Figure 1B) is not present in the human or mouse myotilin sequence.
Figure 1. Alignment of deduced amino acid sequences, and domain structure of chicken myotilin.
A. Alignment of the deduced amino acid sequences with other known myotilin sequences.
B. A schematic diagram of the protein structure shows the serine-rich region (blue box) containing a hydrophobic stretch (not shown) and the two Ig domains (loops) [Salmikangas et al., 1999]. There are eight extra amino acid residues (from 68–75) in chicken myotilin sequence.
Comparison using Pretty software (SeqWeb, a web interface to a core set of GCG programs) shows more divergence of amino acid sequences between chicken and either human myotilin variant 1 or mouse myotilin in the first 250 residues than in the C-terminal half of the protein where the two Immunoglobulin (Ig)-like domains, common elements of actin cross-linking proteins like titin [Von Nadelstadh et al., 2005], reside (Figure 1B).
Localization of YFP tagged chicken myotilin
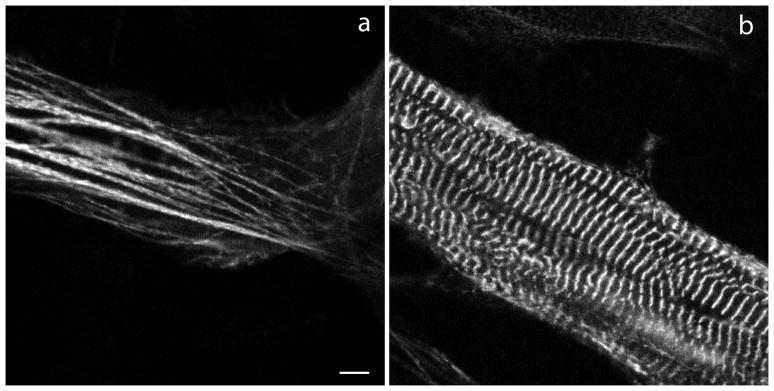
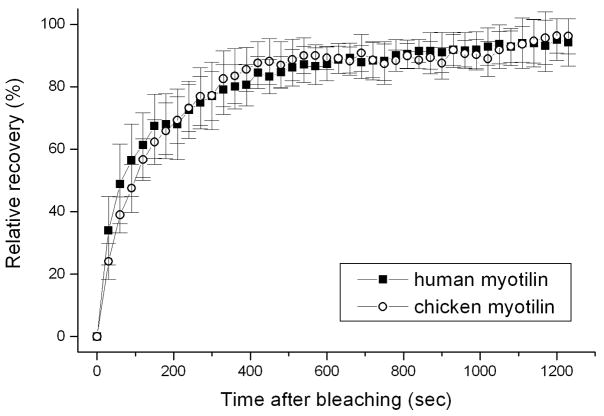
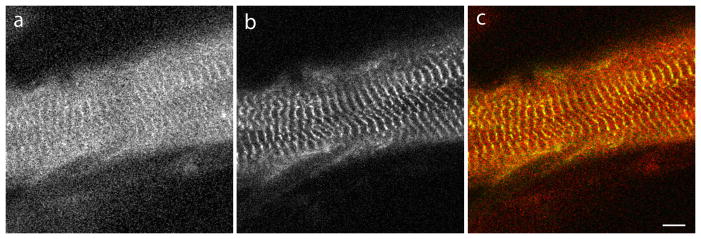
In embryonic quail myotubes, that were transfected on the second day of culture and observed on the fourth culture day, chicken myotilin-YFP was localized in premyofibrils in Z-bodies (Figure 2a), the precursors of muscle Z-bands. At this time point in culture, no mature muscle or contractile activity was present in the myotubes. Beginning on day five when muscle formed in the elongating myotubes, myotilin-YFP localized in Z-bands (Figure 2b), as well as in Z-bodies at the ends of the myotubes where premyofibrils continued to form. This localization mirrors that found following similar transfections of quail myotubes with human myotilin-YFP [Wang et al. 2005]. FRAP measurements demonstrated that the dynamics of chicken myotilin-YFP in Z-bands of quail myotubes was the same as that seen when human myotilin-YFP was expressed in quail myotubes (Figure 3). The mobile fraction for chicken myotilin reached 100% in Z-bands (Figure 3) and in Z-bodies (data not shown), identical to previously reported data for human myotilin-YFP expressed in quail myotubes [Wang et al., 2005].
Figure 2.
Fluorescent images of developing myotubes from embryonic quail muscle in a culture transfected with YFP-chicken myotilin. (a) Myotilin is localized in the premyofibrils at the end of a myotube and (b) in the Z-bands of mature myofibrils at the central region of a myotube. Bar = 5 μm.
Figure 3.
Comparison of the dynamics measured by FRAP of chicken and human myotilin localized in the Z-bands of quail myotubes. The rates and half-lives of exchange of the two isoforms of myotilin are the same in the Z-bands of quail myotubes.
Localization of myotilin fragments in skeletal myotubes
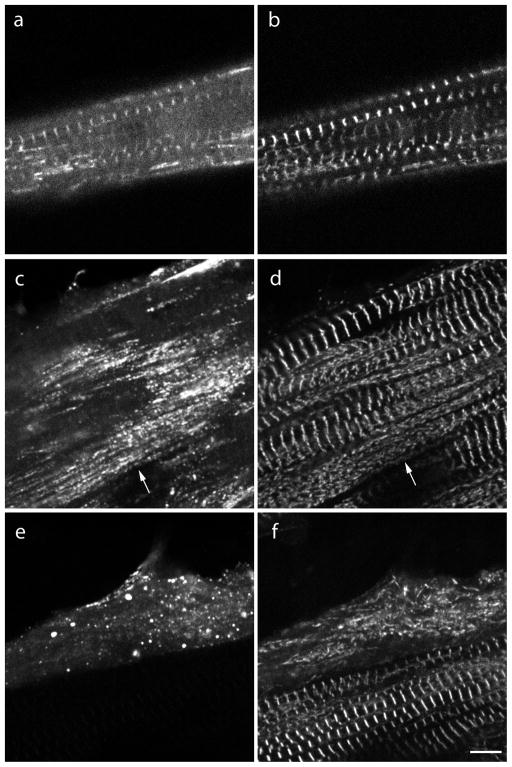
The two halves of the myotilin protein have distinct properties. Mutations leading to myopathies are found in the serine-rich region in the N-terminal half [Selcen, 2011] and the binding sites for Z-band proteins are in the C-terminal half, containing two Ig-like domains through which myotilin forms homodimers [Shalaby et al., 2009]. The two halves, myotilin-N (1–249) and myotilin-C (250–508), also differed in their localization and effects on myofibrils when they were expressed in embryonic quail skeletal myotubes (Figures 4, 5). Myotilin-N, lacking Ig domains, localized weakly in Z-bands and Z-bodies with a high background of unincorporated protein compared with full-length myotilin (Figure 4); however, no disruption of myofibrils was observed in these transfected cells.
Figure 4.
Fluorescence images of quail skeletal muscle cells co-transfected with (a) the N-terminal half of myotilin, and (b) CeFP-alpha-actinin. (c) Merged images of (a) and (b). (a) The N-terminal half of human myotilin (myotilin-N) co-localizes in Z-bands with (b) alpha-actinin, but there is a high background of unincorporated myotilin in the sarcoplasm (a). Bar = 5 μm.
Figure 5.
Fluorescence images of myotubes co-transfected the C-terminal half of YFP-myotilin (a, c, e) and CeFP-alpha-actinin (b, d, f). (a, b) Two to three days post-transfection, myotilin-C co-localized with alpha-actinin in Z-bands of some myotubes. (c, d) In other myotubes, aggregates of myotilin-C (c, arrow) were aligned along myofibrils in myotubes in which alpha-actinin was present in both aggregates (d, arrow) and Z-bands. (e, f) Four days or more after co-transfection, both myotilin-C and alpha-actinin were present as aggregates and Z-bands were absent. (f) Note that the myotube with alpha-actinin incorporation in Z-bands in the lower part of the image did not express myotilin-C.
Bar = 5 μm.
The expression of myotilin-C, in contrast, yielded two types localization after two to three days of transfection: co-localization with alpha-actinin in Z-bands (Figure 5a,b) as was seen after expression full-length myotilin; and localization of myotilin-C in punctate aggregates (Figure 5c,e). In some myotubes, the aggregates of myotilin-C were aligned along myofibrils (Figure 5c). Alpha-actinin, that was co-expressed with the myotilin-C, was present in the same myofibrils in both Z-bands and in punctate aggregates (Figure 5d). In the next one - two days in culture, myotilin-C and alpha-actinin were in punctate aggregates in nearly all myotubes and neither was localized in Z-bands, suggesting myofibril disassembly (Figure 5e,f). In myotubes that were transfected in the same way with myotilin-C, post-staining with phalloidin revealed disorganization of the actin filaments in regions of the myotubes where myotilin-C aggregates were concentrated (Figure 6).
Figure 6.
Fluorescence images of myotubes transfected with (a) YFP-human myotilin-C and then fixed, and stained with (b) rhodamine-phalloidin. (c) Merged images of (a) and (b). The distribution of actin in a sarcomeric pattern was seen in the two myotubes that did not express myotilin-C and in the lower region of the center myotube where the concentration of myotilin-C aggregates was much less than in the disrupted region above. Bar = 5 μm.
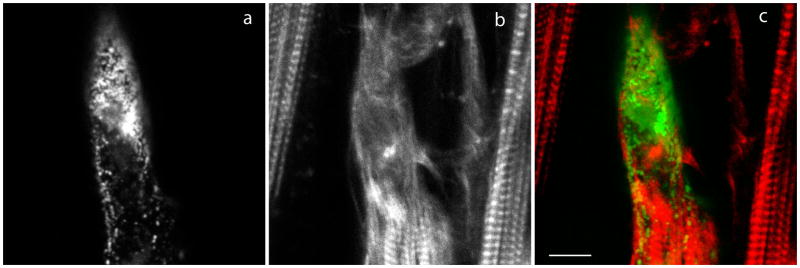
Localization of myotilin fragments in cardiomyocytes
The full-length and N- and C-terminal halves of myotilin had a similar range of localization and effects on myofibrils of cardiomyocytes (Figure 7a–f) as described above for skeletal muscle cells. Myotilin-N localized weakly in Z-bands with a higher background in the sarcoplasm (Figure 7b). Myotilin-C localized in Z-bands (Figure 7c) and also in aggregates in cardiomyocytes in the same culture (Figure 7d). In cardiomyocytes with high concentrations of aggregated YFP-myotilin-C (Figure 7e), myofibrils appeared disrupted and CeFP-alpha-actinin colocalized with myotilin (Figure 7f).
Figure 7.
Fluorescence images of cardiomyocytes transfected with plasmids encoding fragments of human myotilin. (a) YFP-myotilin, (b) N-terminal (YFP-myotilin-N), (c, d) C-terminal (YFP-myotilin-C) of myotilin, (e) YFP-myotilin-C, and (f) same cardiomyocyte as in (e) co-expressing CeFP-alpha-actinin. As in skeletal muscle myotubes, (a) the Z-band localization of full length YFP-myotilin contrasted with (b) a high background of unincorporated YFP-myotilin-N that was present together with Z-band localization of the protein fragment. (c) YFP-myotilin-C localized in Z-bands and also (d) in aggregates in cardiomyocytes in the same culture. (e) In cardiomyocytes with high concentrations of aggregated YFP-myotilin-C myofibrils appeared disrupted and (f) CeFP-alpha-actinin colocalized with myotilin. Bar = 5 μm.
Comparison of the dynamics of fragments and mutations of myotilin expressed in myotubes
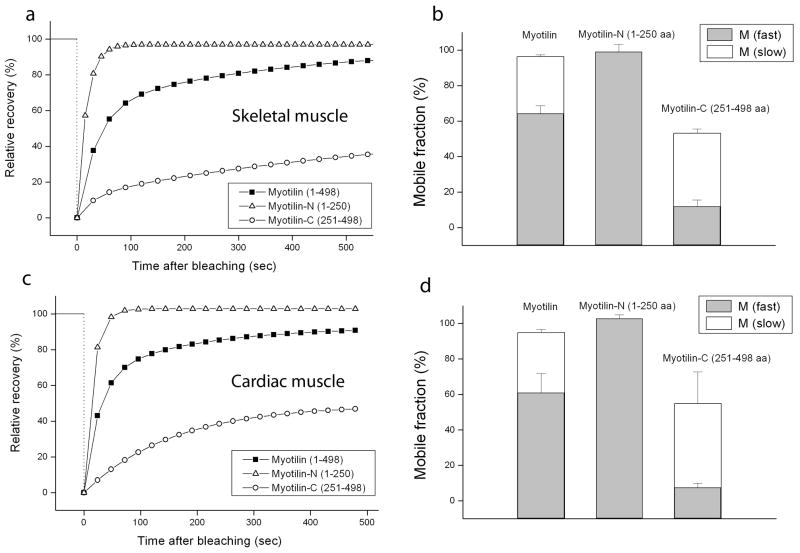
To compare the dynamic exchange of the myotilin fragments in Z-bands in developing myotubes of quail, FRAP analysis was applied to myofibrils that had been transfected with YFP fusions of Myotilin-N- and Myotilin-C (Figure 8, 9). Full-length myotilin-YFP recovered about 80% of its fluorescence in 5 minutes after being bleached, a rate that marks myotilin as a very dynamic protein compared with other myofibrillar proteins like alpha-actinin and actin (Figure 9a) [Wang et al., 2005; Sanger et al., 2009]. The total mobile fraction was about 100% with about 60% fast and 40% slow mobile fractions. The N-terminal half of myotilin exhibited an even faster recovery than full-length myotilin and FRAP analysis showed only one fast 100% mobile fraction (Figure 9a, b) suggesting that without Ig domains, the N-terminal half of the protein lacks the ability to form dimers causing weaker binding to other Z-band proteins. Compared with the N-terminal half and full-length myotilin, the C-terminal half of myotilin showed a significantly slower recovery rate with less than 40% recovery within 5 minutes and decreased mobile fractions compared with Myotilin-N and full-length myotilin (Figure 9a, b). Comparison of FRAP analyses of myotubes versus cardiomyocytes (Figure 9) also revealed similar increases for transfected cardiomyocytes (Figure 9c, d) and myotubes (Figure 9a, b) in fluorescence recovery rates for N-terminal myotilin and decreased rates for C-terminal myotilin compared with full-length myotilin.
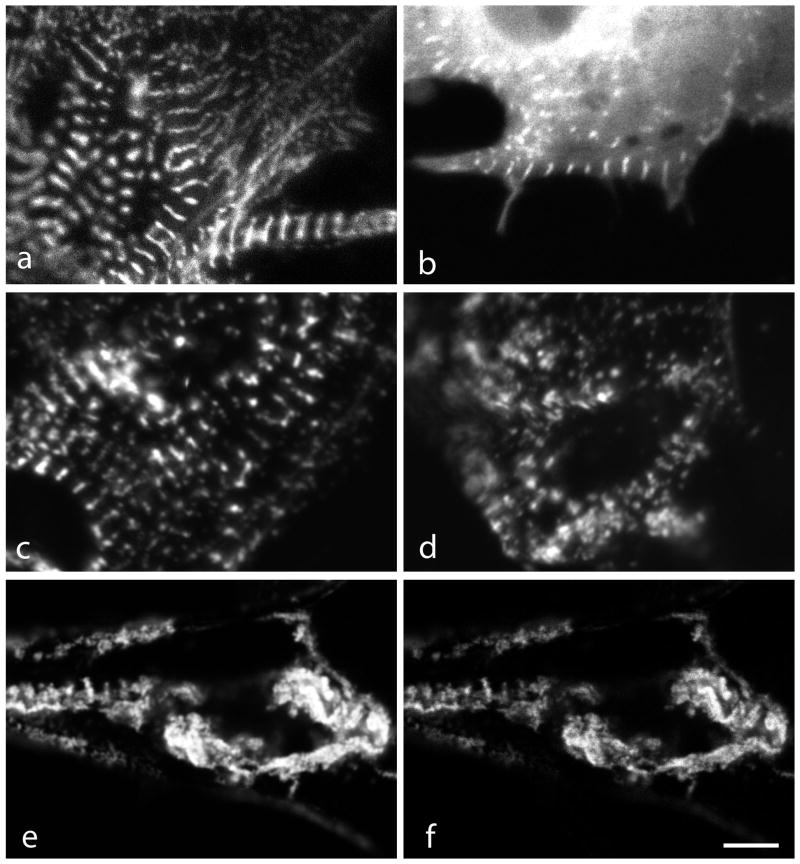
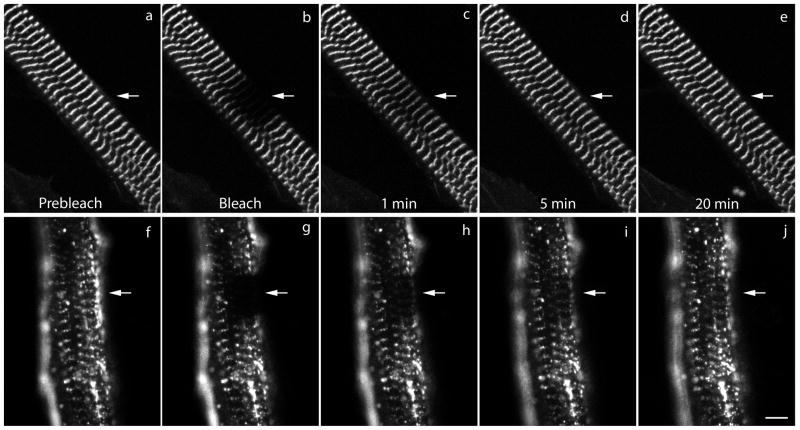
Figure 8.
Images from a FRAP experiment of myotubes expressing (a–e) full-length myotilin or (f–j) myotilin-C. Images (a, f) pre-bleach, (b, g) bleach, (c, h) recovery 1 minute post-bleach, (d, i) recovery 5 minutes post-bleach and (e, j) recovery 20 minutes post-bleach. Bar = 5 μm.
Figure 9.
Recovery after photobleaching in myotubes and cardiomyocytes expressing full-length myotilin or Myotilin-N or Myotilin-C. (a) The average FRAP curve of full-length, N-terminal half and C-terminal half of myotilin in Z-bands of mature myofibrils in myotubes. (b) a comparison of the fast and slow mobile fractions of full-length, myotilin-N and myotilin-C in Z-bands of myofibrils in myotubes. Note that a slow mobile fraction is absent in the recovery of myotilin-N fluorescence. Both mobile fractions of myotilin-C are reduced compared with full-length myotilin. (c) The average FRAP curve of full-length, myotilin-N and myotilin-C in Z-bands of myofibrils in cardiomyocytes and (d) comparison of the fast and slow mobile fractions of full-length myotilin-N and myotilin-C in Z-bands of myofibrils in cardiomyocytes.
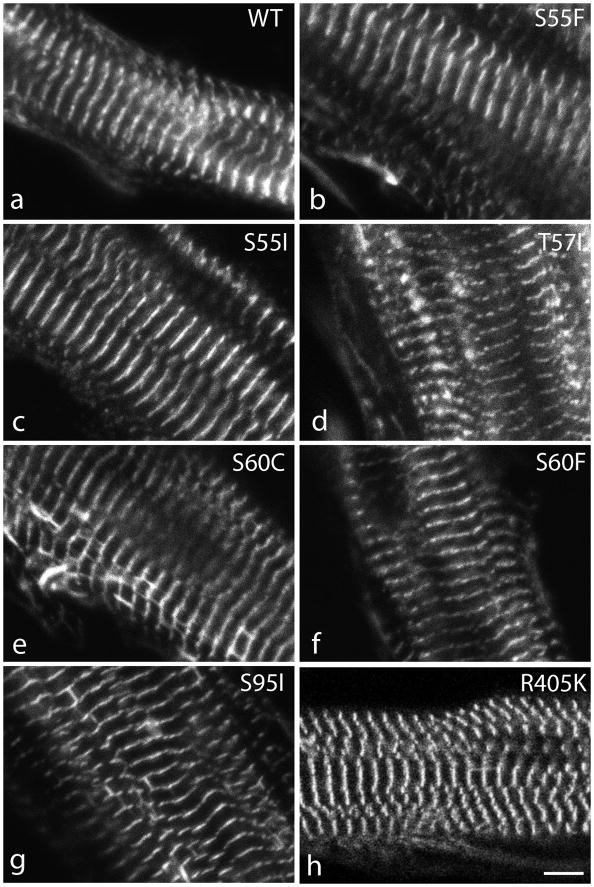
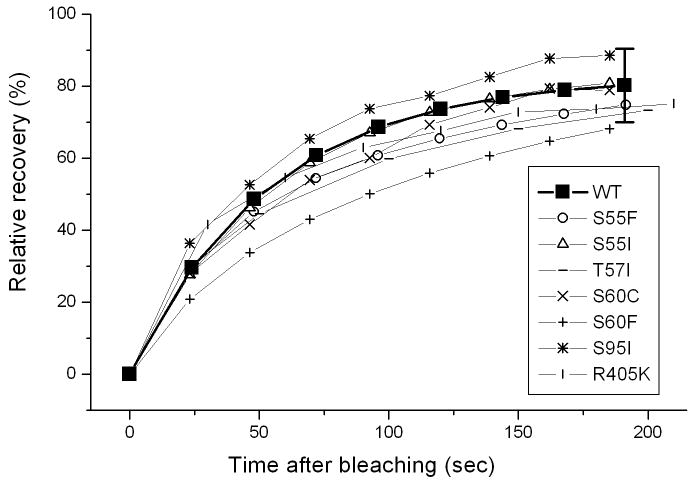
In contrast to the differences in localization and FRAP properties that were seen between expressed full length myotilin and myotilin truncations, no significant differences were detected when human myotilin constructs with single amino acid mutations were expressed in quail myotubes (Figure 10). Myofibrillogenesis occurred normally in all myotubes and each of seven proteins with disease-associated mutations (S55F, S55I, T57I, S60C, S60F, S95I, R405K) localized in the Z-bands of mature myofibrils (Figure 10). Furthermore in FRAP experiments, recoveries for the seven different mutations of myotilin were not significantly different from wild-type myotilin (Figure 11).
Figure 10.
Myotubes expressing wild type myotilin (a) and myotilin with seven different known mutations (b–h). There are no differences between the localization of the wild type myotilin and the myotilin mutants; all are localized in the Z-bands. Bar = 5 μm.
Figure 11.
FRAP curves of wild type myotilin and mutated versions of human myotilin. Seven single amino acid mutations of human myotilin (S55F, S55I, T57I, S60C, S60F, S95I, R405K) were expressed in quail myotubes and localized in the Z-bands of the mature myofibrils. The average curves of the myotilin mutants each fall within the error bars of the wild type myotilin molecules in the Z-bands of mature myofibrils. Two-population t-test analysis revealed that the recoveries for the seven mutations of myotilin were not significantly different from wild type myotilin (95% confidence intervals).
DISCUSSION
Myotilin cDNAs from human, mouse, and some other species have been cloned and sequenced [Salmikangas et al. 1999; Strausberg et al., 2002]. Although a predicted cDNA sequence of chicken myotilin cDNA is available from the databases, to the best of our knowledge, direct cDNA from chicken has not been sequenced. In this study, we have cloned and sequenced the cDNAs of myotilin from chicken heart and skeletal muscle. The nucleotide sequences of myotilin from heart and skeletal muscles are identical (data not shown) suggesting that they are the products of the same Myot gene. The deduced amino acid sequences of chicken myotilin contain 506 amino acid residues whereas human and mouse myotilin contain 498 and 496 amino acid residues respectively. The extra eight amino acid residues in chicken compared to human myotilin variant 1, are located at the N-terminal part of the myotilin peptides as shown in Figure 1B. The percent similarity of myotilin protein compared to human and mouse myotilin is 72 and 70 respectively. The percent identity between chicken and human or chicken and mouse myotilin proteins are 66 and 65 respectively.
Myotilin, together with palladin, and myopalladin, is a member of a family of muscle proteins with Ig domains that function as scaffolds and regulate actin organization [Bang et al., 2001; Mykkanen et al., 2001; Otey et al., 2005; von Nadelstadh et al., 2009]. Myotilin is localized in the Z-bands of cross-striated muscles with many binding partners among which are actin, alpha-actinin, ArgBP2, FATZ/myozenin and filamin [van der Ven et al. 2000; Otey et al., 2005; Gontier et al., 2005; Moza et al., 2007; Moza, 2008; Sanger and Sanger, 2008; von Nadelstadh et al., 2005, 2009; Sanger et al., 2010;]. Although missense mutations in myotilin have been implicated in limb girdle muscular dystrophy type 1A and spheroid body myopathy in humans [Selcen, 2011], it is not clear whether myotilin has any role in myofibrillogenesis. A targeted deletion of the myotilin gene (Myot) does not affect structure and function of muscle in Myot knockout mice [Moza et al., 2007]. This may be due, however, to the ability of the other Z-band proteins with similar Ig-like domains, viz. palladin and myopalladin, to take over the role of the deleted myotilin [Ochala et al., 2009].
Von Nandelstadh et al. [2005] studied the actin binding properties of wild type myotilin and the four myotilin mutants then known to cause myopathies (S55F, T57I, S60C, and S95I), but did not detect any differences between the actin binding properties of the wild type and myotilin mutants. More recently three of these myotilin mutants (S55F, T57I, and S60C) were shown to have slower degradation rates than wild type myotilin [Von Nandelstadh et al., 2011]. How slower degradation rates of myotilin mutants lead to muscle diseases is unknown.
There are now seven different mutations of myotilin that lead to dystrophies in skeletal muscle cells [Salmikangas et al., 1999; Selcen and Engel, 2004; Foroud et al., 2005; Shalaby et al., 2009; Selcen, 2011]. The seven YFP fusion proteins of these myotilin mutants located normally in the Z-bands of mature myofibrils, and we were unable to detect significant differences in dynamic behavior between these myotilin mutants and wild type myotilin measured with FRAP. Perhaps other approaches in the future (e.g., Fluorescence Resonance Energy Transfer or FRET, Stout et al., 2008) may discover differences in the binding properties of the myotilin mutants and their binding partners that lead to myofibrillar instabilities, a hallmark of myotilinopathy [Selcen, 2011].
FRAP is a powerful technique that allows the mobility of proteins between a cytoplasmic pool and a subcellular site to be measured in the setting of the live cell where multiple binding interactions influence a protein’s localization. Full length myotilin is a very dynamic protein recovering 80% of fluorescence within 5 minutes after bleaching. The C-terminal half of the molecule in which all the myotilin binding sites for Z-band proteins are found has a slower recovery of fluorescence indicating a tighter binding of the protein. The myofibril disassembly resulting from expression of myotilin-C may arise when the fragment binds tightly to Z-band partners and competes with full-length myotilin for Z-band localization, impairing the function that the intact myotilin provides and causing disruption of the myofibrils. It may also indicate that the disease-associated serine-rich region in N-terminus is important for normal dynamics and function of myotilin in maintenance of myofibrils.
We found that the C-terminal half of myotilin (aa 251–498) was much less dynamic than that of the full myotilin molecule and the N-terminal half of myotilin (aa 1–250). Its stronger Z-band affinity was accompanied by the formation of aggregates in the cytoplasm of the transfected muscle cells and loss of myofibrils (Figures 5, 6, 11). These results are similar to the effect of a truncated fragment of alpha-actinin: the 27 KD fragment responsible for the actin-binding property of alpha-actinin [Wang et al., 2005]. This fragment of alpha-actinin initially exhibited normal binding to the Z-bands of mature myofibrils, but with time also led to myofibril disassembly. FRAP experiments in myotubes expressing this truncated alpha-actinin revealed a 50% reduction in the recovery of postbleach fluorescence intensity compared wild type YFP-alpha-actinin. We hypothesize that these myotilin and alpha-actinin truncated fragments compete with their full-length counterparts and prevent the normal dynamics of Z-band proteins that are required for the stability and maintenance of Z-bands of the myofibrils. In addition, these truncated fragments are missing parts of the intact molecules that may allow binding to other partners of the Z-bands. The inability to bind these other partners may weaken the Z-band structure and the stability of the mature myofibrils. These truncations of either alpha-actinin or myotilin would be incompatible with embryonic development due to the importance of contractile embryonic hearts and skeletal muscle cells.
In summary, chicken myotilin has properties that are very similar to those of human myotilin molecules. We did not detect any changes in either the localization or in the dynamics of the seven known myotilin mutations in muscle cells (S55F, S55I, T57I, S60C, S60F, S95I, R405K). The C-terminal half of the myotilin molecule, when expressed as a truncated fusion protein, exhibits decreased dynamics in the Z-bands of mature myofibrils in both skeletal and cardiac muscle cells, and induces the disassembly of myofibrils.
Acknowledgments
Grant Sponsor: NIH
The project was supported by Grant Number AR-57063 from NIAMS/NIH, and Grant Number HL080426 from HLBI/NIH (JMS and JWS). JWS is also indebted to the Hendricks Fund for support of this work.
Contributor Information
Jushuo Wang, Email: Wangj@upstate.edu.
Dipak K. Dube, Email: Dubed@upstate.edu.
Balraj Mittal, Email: balrajmittal@gmail.com.
Jean M. Sanger, Email: sangerjm@upstate.edu.
Joseph W. Sanger, Email: sangerjo@upstate.edu.
References
- Bang M-L, Mudry, McElhinny RE, Trombitas AS, Geach K, Yamasaki AJ, Sorimachi R, Granzier H, Gregorio CC, Labeit S. Myopalladin, a novel 145-Kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001;153:413–428. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri GA, Ayoob JC, Turnacioglu KK, Sanger JM, Sanger JW. Use of green fluorescent proteins linked to cytoskeletal proteins to analyze myofibrillogenesis in living cells. Meth Enzymol. 1999;302:171–186. doi: 10.1016/s0076-6879(99)02017-0. [DOI] [PubMed] [Google Scholar]
- Foroud T, Pankratz N, Batchman AP, Pauciulo MW, Vidal R, Miravalle L, Goebel HH, Cushman LJ, Azzarelli B, Horak H, Farlow M, Nichols WC. A mutation in myotilin causes spheroid body myopathy. Neurology. 2005;65:1936–1940. doi: 10.1212/01.wnl.0000188872.28149.9a. FGre. [DOI] [PubMed] [Google Scholar]
- Moreira ES, Wiltshire TJ, Faulkner G, Nilforoushan A, Vainzof M, Suzuki OT, Valle G, Reeves R, Zatz M, Passos-Bueno MR, Jenne DE. Limb-girdle muscular dystrophy type 2G is caused by mutations in the gene encoding the sarcomeric protein telethonin. Nature Genetics. 2000;24:163–166. doi: 10.1038/72822. [DOI] [PubMed] [Google Scholar]
- Moza M, Mologni L, Trokovic R, Faulkner G, Partanen J, Carpen O. Targeted deletion of the muscular dystrophy gene myotilin does not perturb muscle structure or function in mice. Mol Cell Biol. 2007;27:244–252. doi: 10.1128/MCB.00561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moza M. PhD Dissertation. 114. Helsinki University Biomedical Dissertation; 2008. Novel insights on functions of the myotilin/palladin family members. www.doria.fi/bitstream/handle/10024/42601/novelins.pdf?sequence=1. [Google Scholar]
- Mykkanen OM, Gronholm M, Roenty M, Lalowski M, Salmikangas P, Suila H, Carpen O. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–3073. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochala J, Carpen O, Larsson L. Maintenance of muscle mass, fiber size, and contractile function in mice lacking the Z-disc protein myotilin. Upsala J Med Sci. 2009;114:235–241. doi: 10.3109/03009730903276399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Raclin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Inter Natl Rev. 2005;246:31–58. doi: 10.1016/S0074-7696(05)46002-7. [DOI] [PubMed] [Google Scholar]
- Salmikangas P, Mykkanen OM, Grolnholm M, Heiska L, Kere J, Carpen O. Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy. Hum Mol Genet. 1999;8:1329–1336. doi: 10.1093/hmg/8.7.1329. [DOI] [PubMed] [Google Scholar]
- Salmikangas P, van der Ven PF, Lalowski M, Taivainen A, Zhao F, Suila H, Schröder R, Lappalainen P, Fürst DO, Carpén O. Myotilin, the limb-girdle muscular dystrophy 1A (LGMD1A) protein, cross-links actin filaments and controls sarcomere assembly. Hum Mol Genet. 2003;12:189–203. doi: 10.1093/hmg/ddg020. [DOI] [PubMed] [Google Scholar]
- Sanger JM, Sanger JW. The Dynamic Z-bands of striated muscle cells. Science Signal. 2008;1:pe37. doi: 10.1126/scisignal.132pe37. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Wang J, Fan Y, White J, Sanger JM. Assembly and dynamics of myofibrils. Journal of Biomedicine and Biotechnology. 2010;2010 doi: 10.1155/2010/858606. Article ID 858606, 8 pages ( www.hindawi.com/journals/jbb/2010/858606.html. [DOI] [PMC free article] [PubMed]
- Selcen D. Myofibrillar myopathies. Neuromus Disorders. 2011;21:161–171. doi: 10.1016/j.nmd.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcen D, Engel AG. Mutations in myotilin cause myofibrillar myopathy. Neurology. 2004;62:1363–1371. doi: 10.1212/01.wnl.0000123576.74801.75. [DOI] [PubMed] [Google Scholar]
- Shalaby S, Mitsuhashi H, Matsuda C, Minami N, Noguchi S, Nonaka I, Nishino I, Hayashi YK. Defective myotilin homodimerization caused by a novel mutation in myot exon 9 in the first Japanese limb girdle muscular dystrophy 1a patient. J Neuropathol Exp Neurol. 2009;68:701–707. doi: 10.1097/NEN.0b013e3181a7f703. [DOI] [PubMed] [Google Scholar]
- Stout AL, Wang JJ, Sanger JM, Sanger JW. Tracking changes in Z-band organization during myofibrillogenesis with FRET imaging. Cell Motil Cytoskeleton. 2008;65:353–367. doi: 10.1002/cm.20265. [DOI] [PubMed] [Google Scholar]
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA. 2002;99 (26):16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven PF, Wiesner S, Salmikangas P, Auerbach D, Himmel M, Kempa S, Hayess K, Pacholsky D, Taivainen A, Schröder R, Carpén O, Fürst DO. Indications for a novel muscular dystrophy pathway. Gamma-filamin, the muscle-specific filamin isoform, interacts with myotilin. J Cell Biol. 2000;151:235–248. doi: 10.1083/jcb.151.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Nandelstadh P, Groenholma M, Moza M, Lambergc A, Savilahtic H, Carpen O. Actin-organising properties of the muscular dystrophy protein myotilin. Exp Cell Res. 2005;310:131–139. doi: 10.1016/j.yexcr.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Von Nadelstadh P, Ismail M, Gardin C, Suila H, Zara I, Belgrano A, Valle G, Carpej O, Faulkner G. A class III PDZ binding motif in the myotilin and FATZ families binds enigma family proteins: a common link for Z-Disc myopathies. Mol Cell Biol. 2009;29:822–834. doi: 10.1128/MCB.01454-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Nadelstadh P, Soliymani R, Baumann M, Carpen O. Analysis of myotilin turnover provides mechanistic insight into the role of myotilinopathy-causing mutations. Biochem J. 2011;436:113–121. doi: 10.1042/BJ20101672. [DOI] [PubMed] [Google Scholar]
- Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of Z-band proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton. 2005;61:34–48. doi: 10.1002/cm.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sanger JM, Kang S, Thurston H, Abbott LZ, Dube DK, Sanger JW. Ectopic expression and dynamics of TPM1alpha and TPM1kappa in myofibrils of avian myotubes. Cell Motil Cytoskeleton. 2007;64:767–776. doi: 10.1002/cm.20221. [DOI] [PubMed] [Google Scholar]
- Wang J, Thurston H, Essandoh E, Otoo M, Han M, Rajan A, Duibe S, Zajdel RW, Sanger JM, Linask KK, Dube DK, Sanger JW. Tropomyosin expression and dynamics in developing avian embryonic muscles. Cell Motil Cytoskeleton. 2008;65:379–392. doi: 10.1002/cm.20267. [DOI] [PubMed] [Google Scholar]
- Zajdel RW, Denz CR, Lee S, Dube S, Ehler E, Perriard E, Perriard J-C, Dube DK. Identification, characterization, and expression of a novel alpha-tropmyosin isoform in developing chicken. J Cell Biochem. 2003;89:427–439. doi: 10.1002/jcb.10504. [DOI] [PubMed] [Google Scholar]