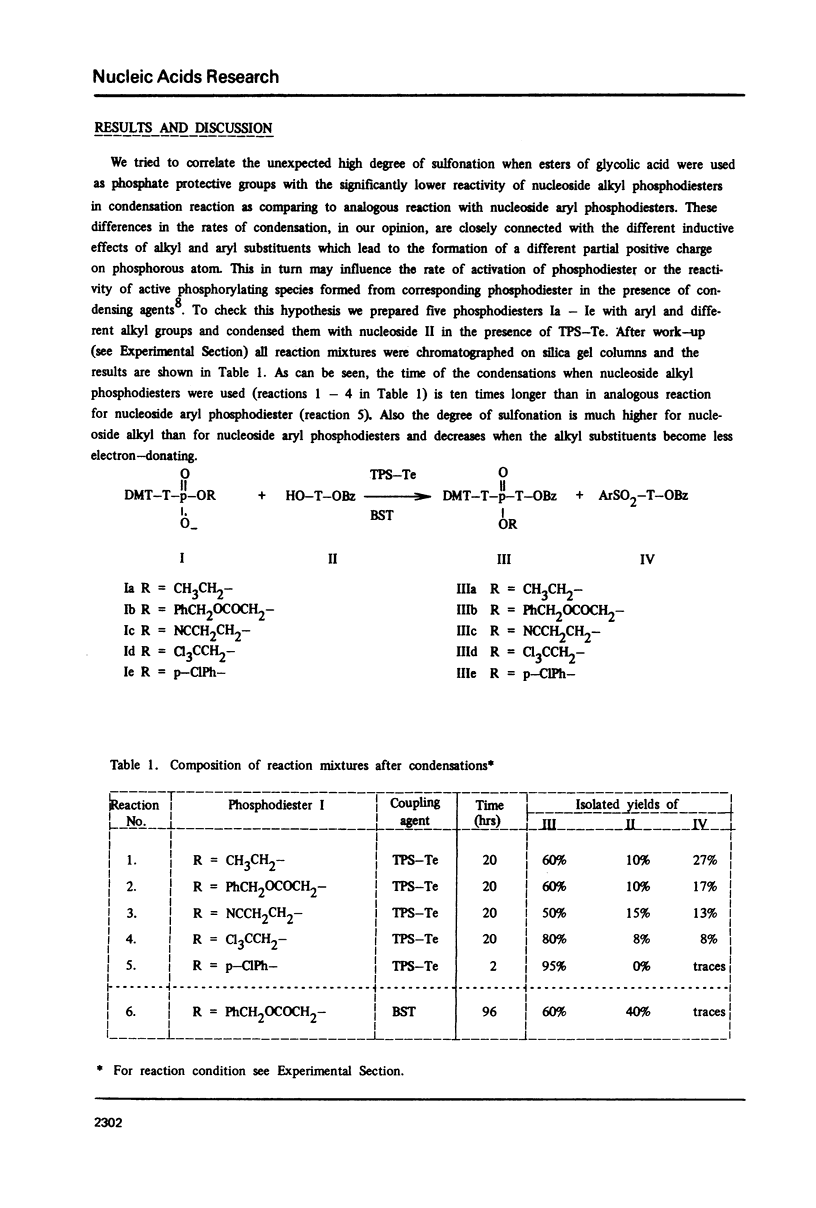
Abstract
The sulfonation of nucleosidic component, a side reaction during phosphotriester bond formation, as a function of the reactivity of the condensing agents and the kind of substituents in the starting phosphodiester is discussed. It was found that in the coupling reaction of nucleoside alkyl phosphodiesters, the degree of sulfonation of the nucleosidic component was very high; while under the same conditions when the aryl group was present in the corresponding phosphodiester, this side reaction practically did not occur.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Katagiri N., Itakura K., Narang S. A. The use of arylsulfonyltriazoles for the synthesis of oligonucleotides by the triester approach. J Am Chem Soc. 1975 Dec 10;97(25):7332–7337. doi: 10.1021/ja00858a021. [DOI] [PubMed] [Google Scholar]
- Lohrmann R., Söll D., Hayatsu H., Ohtsuka E., Khorana H. G. Studies on polynucleotides. LI. Syntheses of the 64 possible ribotrinucleotides derived from the four major ribomononucleotides. J Am Chem Soc. 1966 Feb 20;88(4):819–829. doi: 10.1021/ja00956a039. [DOI] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]



