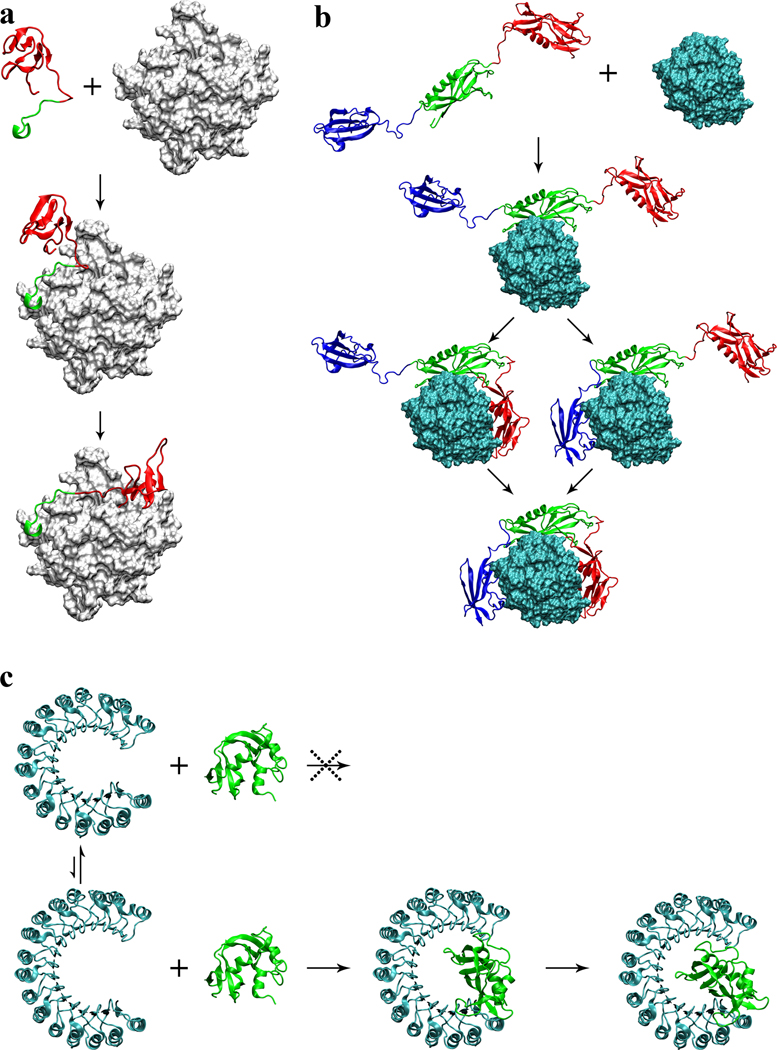
Figure 3.
Proposed association mechanisms of three complexes. (a) Hirudin/thrombin association. First the acidic C-terminal tail (in green) of hirudin docks to the fibrinogen recognition site on thrombin (gray surface); then the N-terminal domain (in red) coalesces around the active site. (b) Streptokinase/plasmin association. First the β domain (in green) of streptokinase docks to plasmin (cyan surface); subsequently the α and γ domains (in red and blue, respectively) coalesce around plasmin to form a tight complex. (c) Ribonuclease inhibitor/ribonuclease A association. Ribonuclease inhibitor (in cyan) undergoes conformational fluctuations, resulting in variations in the horseshoe opening. Small opening prevents the binding of ribonuclease A (in green); large opening allows deep insertion of the enzyme, and subsequently contraction leads to a tight complex.