Figure 4. The GPDR mutated ESA peptide can inhibit Sirt1 activity in vivo.
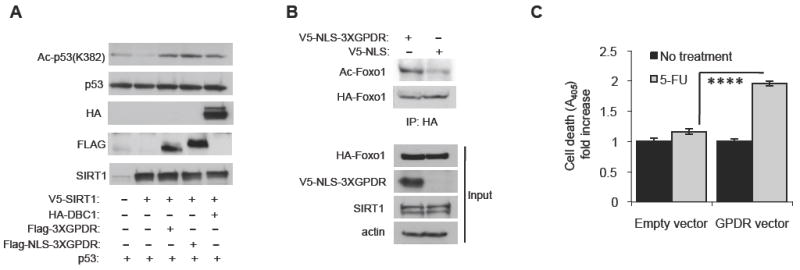
(A) The GPDR mutated ESA peptide inhibits deacetylation of p53 by Sirt1 in H1299 cells. Expression vectors for p53 and V5-tagged Sirt1 were co-transfected with a 10-fold molar excess of an expression vector for HA-tagged DBC1, Flag-tagged 3XGPDR (Flag-3XGPDR) or Flag-tagged 3XGPDR with a nuclear localization signal (Flag-NLS-3XGPDR). Levels of acetylated p53 were visualized by antibody specific for acetylated K382 in p53.
(B) The GPDR mutated ESA peptide inhibits deacetylation of Foxo1 by Sirt1 in the prostate cancer cell line DU145. An expression vector for HA-tagged Foxo1 was co-tranfected with an expression vector for either V5-tagged-NLS-GPDR (3X) peptide or V5-tagged NLS without GPDR. Acetylation of Foxo1 was visualized by immunoblotting with an antibody specific for acetylated lysine after HA-Foxo1 was immunoprecipitated with anti-HA antibody.
(C) The GPDR mutated ESA peptide restores sensitivity to the chemotherapeutic agent 5-FU. DU145 cells were co-transfected with an expression vector for V5-tagged-NLS-GPDR or control and treated with 20 μM 5-FU for two days before apoptosis levels were measured. Results are expressed as the mean ± s.e.m. ****, p<0.0001 between empty and GPDR vectors.
