Figure 2. Key role of the IFN-γ-pathway in LMP2 expression in normal myometrium.
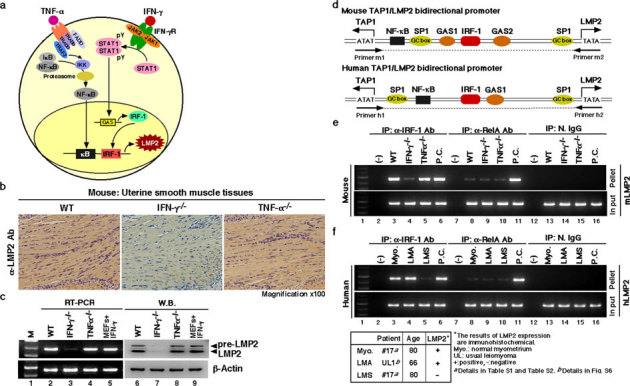
(a) Key role of the signaling pathway on LMP2 expression. (b) Immunohistochemical experiments with myometrium tissue sections derived from wild-type, IFN-γ-deficient, and TNF-α-deficient mice (2 months old) were carried out. (magnification x100) The results revealed that the IFN-γ signaling cascade was required for basal LMP2 expression. (c) Western blotting and RT-PCR experiments with myometrium tissue sections derived from wild-type and IFN-γ- and TNF-α-deficient mice (2 months old) were also performed. The results showed that IFN-γ-deficient mice had markedly reduced LMP2 levels in myometrium tissues. These findings support the notion that the IFN-γ pathway plays a key role in basal LMP2 expression. (d) Schematic representation of the LMP2/TAP1 bidirectional promoter, including NF-κB and IRF-1 binding sites. Chromatin immunoprecipitation (ChIP) analysis with antibodies against RelA (NF-κBp65) and IRF-1 was carried out. (e) ChIP assays showing that although mouse genomic DNA of the Lmp2 enhancer/promoter region was markedly amplified using immunoprecipitated TNF-α-deficient myometrium tissue with anti-IRF-1 antibody, amplified products were not detected using immunoprecipitated IFN-γ-deficient myometrium tissue with anti-IRF-1 antibody. The mouse genomic DNA of the LMP2 enhancer/promoter region was unclearly amplified using the immunoprecipitated materials with anti-RelA antibody. Positive control (P.C.): IFN-γ-treated mouse embryonic fibroblasts (lane 6), TNF-α-treated mouse splenocytes (lane 11). (f) ChIP assays showing that although human genomic DNA of the LMP2 enhancer/promoter region was markedly amplified using immunoprecipitated LMA tissue as well as normal myometrium tissue with anti-IRF-1 antibody, no-DNA amplification was detected in the immunoprecipitated LMS sample with anti-IRF-1 antibody. The DNA of the LMP2 enhancer/promoter region was not clearly amplified using any immunoprecipitated myometrium tissue materials with anti-RelA antibody. Positive control (P.C.): IFN-γ-treated mouse embryonic fibroblasts (lane 6), TNF-α-treated mouse splenocytes (lane 11). N.IgG, normal rabbit anti-serum was used as a negative control antibody. The experiments were performed four times with similar results.
