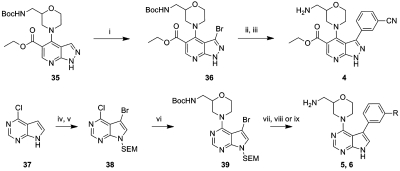
Scheme 1.
Reagents and conditions: (i) NBS, DMF, rt, 77%; (ii) PdCl2(dppf)·CH2Cl2, 3-cyanophenylboronic acid, Na2CO3, DME/H2O, 140 °C, 2 h, microwave, 48%; (iii) TFA, MeOH, 80 °C, 62%; (iv) NBS, CH2Cl2, rt, 83%; (v) NaH, SEM-Cl, DMF, 0 °C to rt, 93%; (vi) Boc-amine, TEA, n-BuOH, 100 °C, 68%; (vii) Pd(PPh3)4, phenylboronic acid, Na2CO3, DME/H2O, 120 °C, 30 min, microwave, 49–59%; (viii) TBAF, ethane-1,2-diamine, DMF, 60 °C, 7 h and then TFA, MeOH, 80 °C, 21%; (ix) TBAF, ethane-1,2-diamine, DMF, 60 °C, 7 h and then 4 M HCl–dioxane, MeOH, 24 h, rt, 17%.