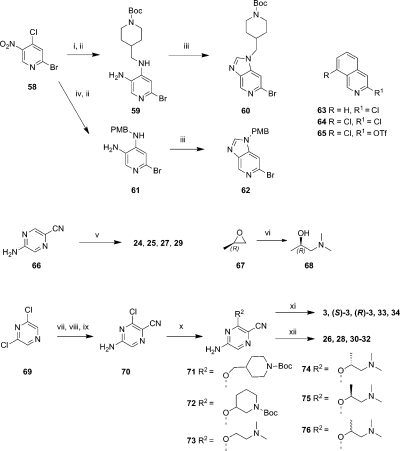
Scheme 4.
Reagents and conditions: (i) 4-(aminomethyl)piperidine-1-carboxylate, TEA, MeCN, 1.5 h, rt, 93%; (ii) SnCl2·2H2O, EtOH, 70 °C, 2 h, 88%; (iii) (EtO)3CH, Ac2O, 100 °C, 18 h, 40%–100%; (iv) 4-methoxybenzylamine, TEA, MeCN, 1.5 h, rt, 77%; (v) aryl halide (ArCl for 24, 25, 29; ArI for 27), Pd(OAc)2, (±)-BINAP, NaOtBu, DMF/Tol, 150 °C, 30 min, microwave and then MP-TsOH column, 3–41% (11% for 25 including Boc deprotection); (vi) NHMe2 (40% in H2O), 0–20 °C, 2 h 40%; (vii) aq NH3 (28%), 100 °C, o/n, 91%; (viii) NBS, CH2Cl2, 0 °C, 1 h, 42%; (ix) CuI, 18-crown-6, Pd(PPh3)4, KCN, DMF, reflux, 3 h, 82%; (x) NaH (60% in oil), dioxane, aminoalcohol, 100 °C, o/n, 14–32%; (xi) Pd(OAc)2, (±)-BINAP, NatBu, DMF/toluene, microwave, 140–150 °C, 30 min, or Pd2(dba)3, Xantphos, Cs2CO3, toluene, microwave, 140 °C, 45 min (2–45%); (xii) Pd(OAc)2, (±)-BINAP, NatBu, DMF/Tol, microwave, 140–150 °C, 30 min, or Pd2(dba)3, Xantphos, Cs2CO3, toluene, microwave, 140 °C, 45 min followed by TFA, 80 °C, 30 min or TFA, CH2Cl2, rt, 1 h MP-TsOH column (2–26%).