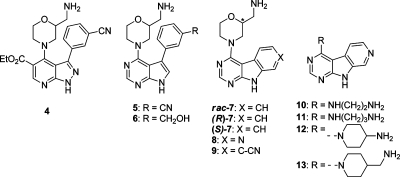
Table 1. Inhibition of CHK1 and CHK2 by Bicyclic and Tricyclic Compounds.
| CHK1 Inhibition IC50 (μM)a | CHK2 Inhibition IC50 (μM)a | Selectivity (CHK2/CHK1)b | LE (kcal mol–1 non-H atom–1)c | |
|---|---|---|---|---|
| 2 | 1.0 (0.86, 1.2) | 50 (26, 74) | 50 | 0.38 |
| 4 | 1.5 (1.3, 1.8) | n.d.d | 0.27 | |
| 5 | 3.2 (2.2, 4.2) | 28 (13, 43) | 9 | 0.31 |
| 6 | 0.43 (0.26, 0.60) | 3.4 (±1.16)e | 8 | 0.36 |
| rac-7 | 3.2 (2.4, 3.9) | 12 (11, 13) | 4 | 0.37 |
| (R)-7 | 3.0 (2.9, 3.2) | 13 (11, 16) | 4 | 0.37 |
| (S)-7 | 2.3 (1.55, 3.0) | 3.5 (2.3, 4.6) | 2 | 0.38 |
| 8 | 0.88 (±0.21) e | 19 (16, 23) | 22 | 0.40 |
| 9 | 3.7 (3.2, 4.2) | 10 (9.3, 11) | 3 | 0.33 |
| 10 | 1.7 (1.7, 1.8) | 23 (22, 24) | 14 | 0.47 |
| 11 | 1.7 (1.7, 1.8) | 11 (8.1, 14) | 6 | 0.45 |
| 12 | 0.67 (0.53, 0.80) | 16 (10, 21) | 24 | 0.43 |
| 13 | 0.29 (±0.02)e | 12 (6.8, 16) | 41 | 0.43 |
IC50 determined in a dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA).(33) Mean of two independent determinations; individual values in parentheses. Standard inhibitor staurosporine gave CHK1 IC50 = 2.1 (±1.8) nM and CHK2 IC50 = 27 (±8) nM.
Ratio of IC50 values (CHK2/CHK1).
Ligand efficiency (LE) calculated using LE = [−1.4 log10(IC50 (M))]/(number of heavy atoms).(37)
Not determined.
Mean (±SD) of at least three independent determinations.