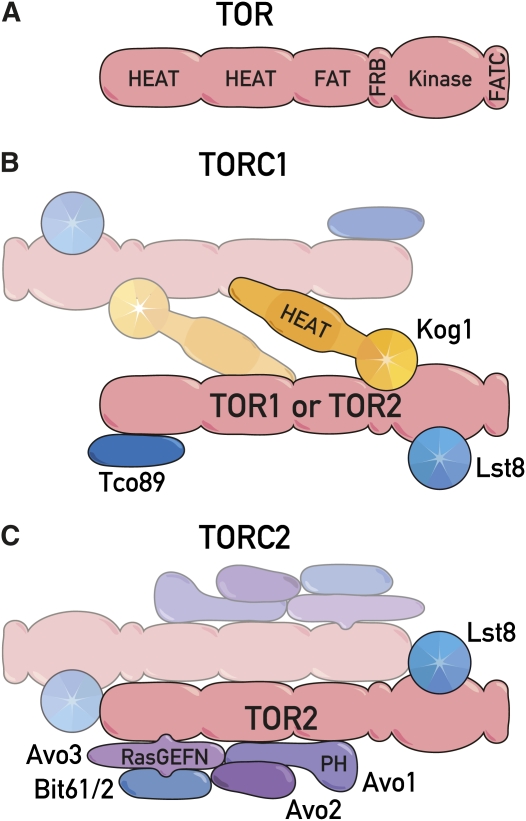
Figure 1.
(A) Conserved domain structure of TOR. The N-terminal half of TOR is composed of two blocks of ∼20 HEAT repeats, 40 aa that form pairs of interacting antiparallel α-helices. The ∼500-aa FAT (FRAP-ATM-TRRAP) domain contains modified HEAT repeats. Missense mutations in the ∼100-aa FRB (FKBP12-rapamycin-binding) domain confer complete resistance to rapamycin. The kinase domain phosphorylates Ser/Thr residues in protein substrates, but at the sequence level resembles the catalytic domain of phosphatidylinositol kinases. The ∼35-aa FATC domain is always found C-terminal to the FAT domain and is essential for kinase activity. (B) Composition of TOR complex 1. TORC1 is ∼2 MDa in size and contains Kog1, Tco89, Lst8, and either TOR1 or TOR2. The HEAT repeats found in Kog1 and the seven-bladed propellers of the WD-40 repeats found in Kog1 and Lst8 are depicted. The binding of Kog1 to TOR is complex, involving multiple domains on each protein. Lst8 binds to the kinase domain of TOR. Each component is likely present in two copies. (C) Composition of TOR complex 2. TORC2 is ∼2 MDa in size and contains Avo1, Avo2, Avo3, Bit61, and/or its paralog Bit2, Lst8, and TOR2 but not TOR1. The RasGEFN domain of Avo3 and the PH domain of Avo1 are indicated. Each component is likely present in two copies.