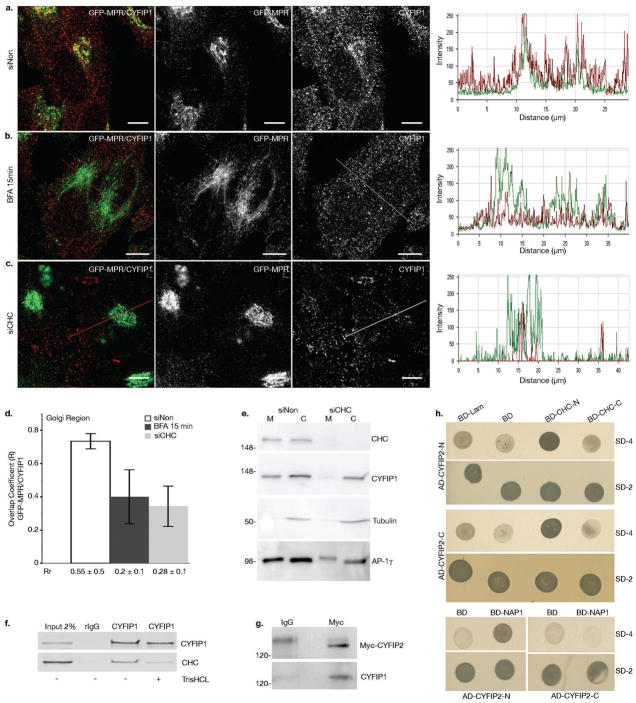
Figure 2. CYFIP1 and CYFIP2 interact with CHC and CYFIP1 recruitment to the TGN is regulated by ARF1.
(a-d) HeLa cells stably expressing GFP-MPR were treated with (a) control siRNA or (c) siRNAs to deplete CHC or (b) incubated with 5 μg ml−1 BFA for 15 min. Cells were then labeled with anti-CYFIP1 (red), and (d) the overlap (R) and Pearson correlation (Rr) coefficients between GFP-MPR and CYFIP1 in the TGN region were quantified for each condition (20 cells from n = 3 independent experiments were analyzed per condition; data represent the mean ± s.d.). Scale bars, 10 μm. (e) The membrane (M) and cytosolic (C) fractions of HeLa cells incubated either with siNon or with siCHC were analyzed by western blot (n = 3 independent experiments). (f) COS-7 cell lysates were incubated with anti-CYFIP1 or with control pre-immune rabbit IgG. Beads were washed with buffer with or without 0.5 M TrisHCl (pH 7.4), which induces clathrin cage depolymerization. The presence of CHC and CYFIP1 in the immunoprecipitates was determined by western blotting using the corresponding antibodies. CHC was co-immunoprecipitated with CYFIP1 only in the absence of TrisHCl (n = 3 independent experiments). (g) Lysates of HeLa cells transiently expressing myc-tagged CYFIP2 were incubated with anti-myc or control mouse IgGs and the immunoprecipitates were analyzed by western blotting. Full scans of all gels are shown in Supplementary Information, Fig. S9. (h) The N-terminal (AD-CYFIP2-N, AA 2–623) and the C-terminal domains C (AD-CYFIP2-C, AA 674–1299) of CYFIP2 were expressed as fusions with GAL4AD (pGADT7). The N-terminal (BD-CLC-N, AA 1–690) and the C-terminal (BD-CLC-C, AA 821–1679) halves of clathrin heavy chain, as well as (i) full-length Nap1 were fused to the DNA-BD (pGBKT7), and co-expressed with the GAL4AD containing plasmids. Interactions were detected by growth on agar plates lacking leucine and tryptophane (SD-2), or adenine, histidine, leucine and tryptophane (SD-4). Plasmids expressing either fusion of lamin C to the DNA-BD (BD-Lam) or DNA-BD alone (BD) were used as negative controls. N = 3 independent experiments.