Fig. 5.
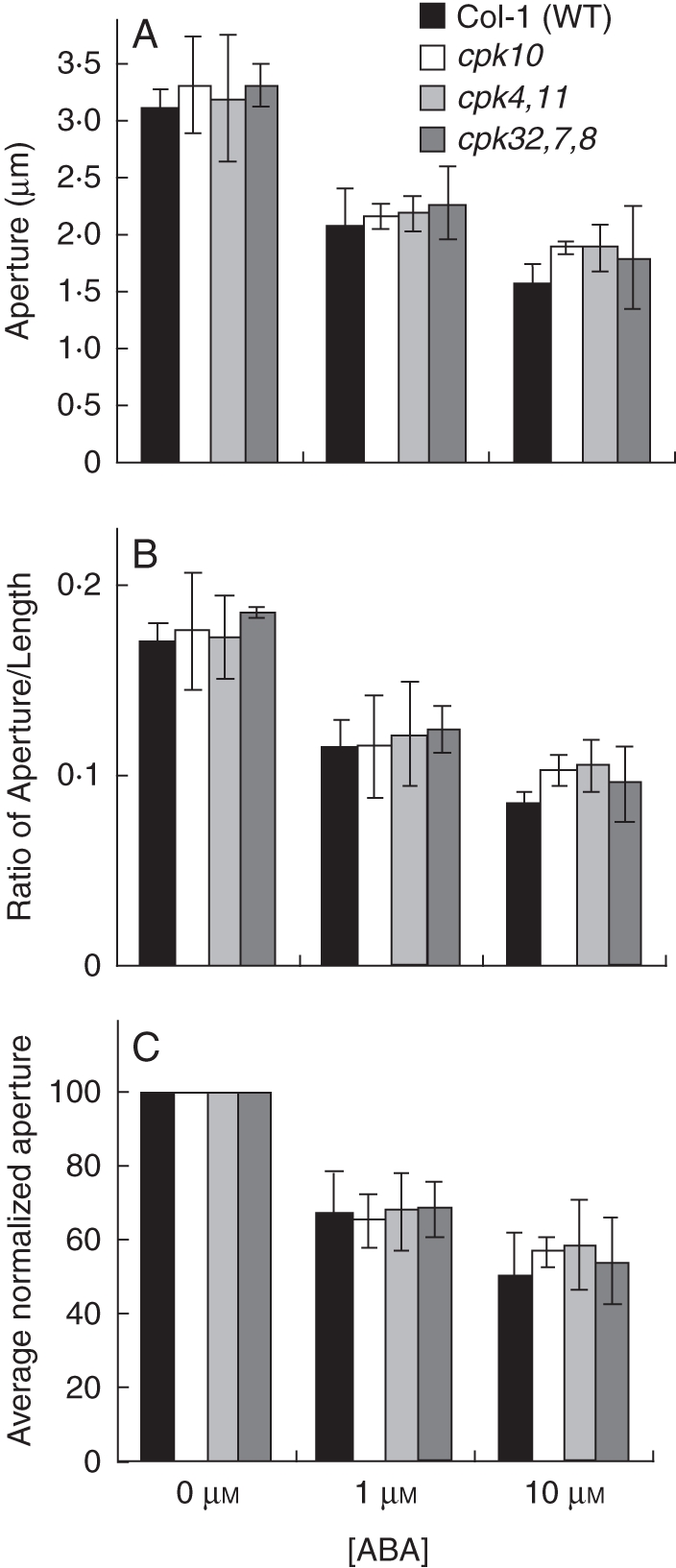
ABA-induced stomatal closure was not dramatically impaired in cpk10, cpk4cpk11 and cpk32cpk7cpk8 compared with Col-1 wild type. Whole leaves were pre-incubated in stomatal-opening buffer (10 mm KCl, 7·5 mm iminodeactic acid, 10 mm MES, pH 6·2) for 2 h and then incubated for an additional hour with 0, 1 or 10 µm ABA. Stomatal aperture (A) and ratio (width : length) (B) were then measured. In (C) the average normalized stomatal apertures from (A) are shown. Experiments were performed double blind with both genotype and ABA concentration unknown; n = 3 experiments with 30 stomata analysed per condition in each experiment (90 stomatal apertures analysed per bar in the graphs). Error bars represent ± s.e. of the mean relative to n = 3 experiments. Plants were grown at approx. 95 % humidity and were subjected to 24 h of high (>98 %) humidity prior to experimentation. ABA-dependent stomatal movement imaging analyses (Valerio, 2007) were performed following the protocol from Kwak et al. (2002). Rosette leaves from 4- to 5-week-old plants were excised and the curvature of each leaf was gently inverted using a finger so that the abaxial side did not form any air pockets when floating on solution during incubation. Using forceps the whole leaf was placed with the abaxial side down in 4 mL of stomatal-opening buffer (10 mm KCl, 7·5 mm iminodeactic acid, 10 mm MES, pH 6·2) in a Petri dish (35 × 10 mm). Leaves were incubated in stomatal-opening buffer for 2 h under white light (200 µE). Then, the stomatal-opening buffer was removed via a pipette and replaced with 4 mL stomatal-opening buffer containing 0 µm, 1 µm or 10 µm ABA and the leaves were incubated under white light for one more hour. The abaxial epidermis of each leaf was peeled using forceps and placed on a glass coverslip with approx. 200 µL of the treatment buffer. Experiments were performed double-blinded. Both genotype identity and ABA concentrations were unknown to the experimenter. Data are from Valerio (2007).
