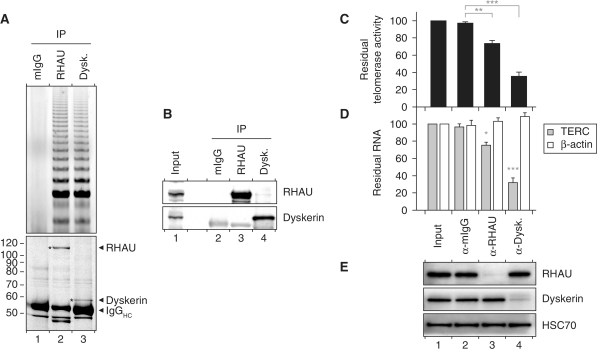
Figure 6.
Association of RHAU with telomerase activity. (A) TRAP assay of immunopurified endogenous RHAU and dyskerin RNP complexes. RHAU or dyskerin RNP complexes were enriched by immunoprecipitation and a fraction of the immunopurified RNP was essayed for telomerase activity by the TRAP assay. Mouse IgGs (mIgGs) served as a control to assess non-specific interactions to the antibody matrix. A fraction of the immunopurified RNP was separated by SDS–PAGE. The Coomassie stained gel was scanned on a LI-COR Odyssey infrared imaging system. Positions of the immunoprecipitated proteins as well as the immunoglobulin heavy chains (IgGHC) are indicated at the right. Positions and sizes (kDa) of marker proteins are shown at the left. (B) Western blot analysis of the immunopurified RNP complexes assayed for telomerase activity. (C) qTRAP analysis of the residual telomerase activity present in immunodepleted HEK293T cell lysates. Data represent the mean ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA and the Bonferroni t-test. *P < 0.05; **P < 0.01; ***P < 0.001. (D) RT–qPCR analysis of the residual levels of TERC and β-actin RNAs in immunodepleted HEK293T cell lysates. The RNA signal was normalized to GAPDH. Data represent the mean ± SEM of three independent experiments. Statistical significance was determined by one-way ANOVA and the Bonferroni t-test. (E) Western blot analysis of input and immunodepleted HEK293T cell lysates. Heat shock protein 70 cognate (HSC70) was used as a loading control.