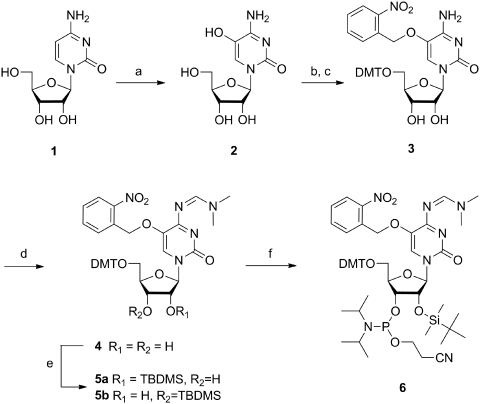
Scheme 1.
(a) 1. Br2, H2O, 0°C; 2. sym-collidine, 40°C, 2 h; 64%; (b) 4,4′-dimethoxytriphenyl chloride, AgNO3, pyridine, 3.5 h, rt; (c) 2-nitrobenzyl bromide, K2CO3, NaI, acetone, 60°C, 3 h, 64% over two steps; (d) N,N-dimethylformamide dimethyl acetal, DMF, 60°C, 1 h, 99%; (e) tert-butyldimethylsilyl chloride, AgNO3, pyridine, THF, 2 h, 50% for 5a; (f) 2-cyanoethyl diisopropylchlorophosphoramidite, N-ethyldiisopropylamine, THF, rt, 16 h, 71%. DMT = 4,4′-dimethoxytriphenyl, TBDMS = tert-butyldimethylsilyl.