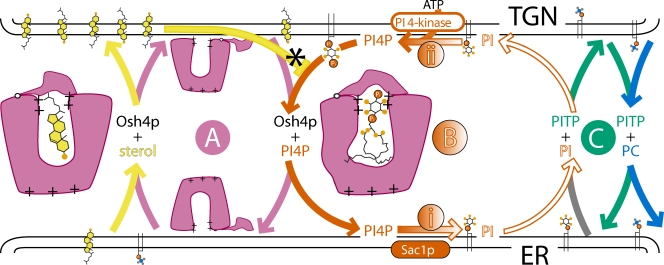
Figure 1.
Cycling by two lipid transfer proteins and a “futile cycle” of PI(4)P production can drive sterol traffic. Osh4p can exist in one of three states: empty, bound to sterol, or bound to phosphatidylinositol 4-phosphate PI(4)P, altering its structure slightly. PI(4)P synthesis potentially drives sterol up a concentration gradient. (A) Osh4p (pink) exchanges sterol forward (yellow) for PI(4)P back (red). (B, i) PI(4)P is dephosphorylated by Sac1p. (C) PITP (PI/PC transfer protein, green; detail omitted) exchanges PC (blue) into the ER and PI (white) forward. (B, ii) PI is phosphorylated. For the cost of one ATP, PI goes round an apparently futile cycle. A key point is that after bringing PI(4)P back to the ER, Osh4p will most likely fill up with sterol. However, extraction of PI(4)P at the TGN is limited because sterol competes for binding with PI(4)P (asterisk). An ER-TGN cycle is shown, but an ER-PM cycle would be similar, possibly using a different ORD protein.