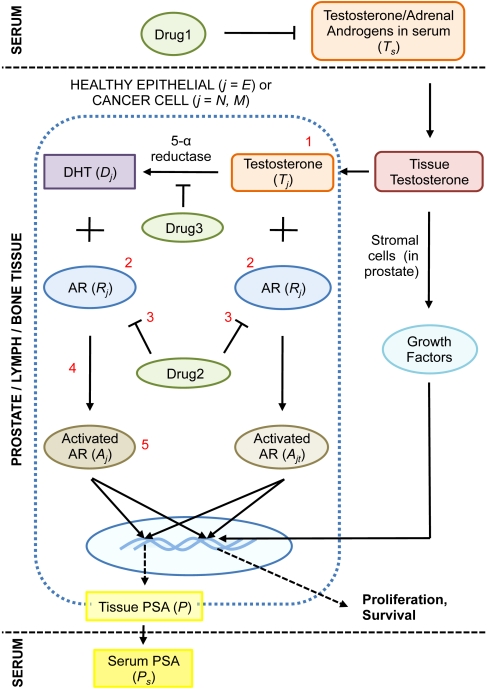
Fig. 1.
The dynamics of intracellular androgen biology governing prostate cancer (CaP) growth is shown. Briefly, testosterone is synthesized by the testes (not shown) and released into the circulation. From here, it enters the prostate and is taken up by prostatic epithelial cells (E), androgen-dependent cancer cells (N), and castrate-resistant cancer cells (M). Testosterone is converted into DHT by the action of 5α-reductase. Both testosterone and DHT subsequently bind and activate androgen receptors, a process mediated by additional cofactors, resulting in proliferative and survival signal modulation. PSA production is also androgen-dependent and related to cancer volume. Growth factors expressed by stromal cells at the site of tumor growth include: IGF-I, VEGF, bFGF, FGF7, TGF-β. However, given the paucity of data, we do not include the role of growth factors that might be secreted by the stroma on cancer cell survival or death explicitly in our model. Instead, it may be assumed that this is implicitly accounted for in the fits of prostate growth. In our model, castration-resistant cancer cells arise as a result of random mutations that occur when androgen-dependent cancer cells proliferate. Several mutations resulting in castration-resistance are numbered in red (1: local production of androgens, 2: up-regulated AR expression, 3: drug 2-induced activation of AR, 4: lowered AR activation threshhold, 5: constitutively activated AR). Finally, the actions of three types of drugs used to treat CaP are shown in green ovals.