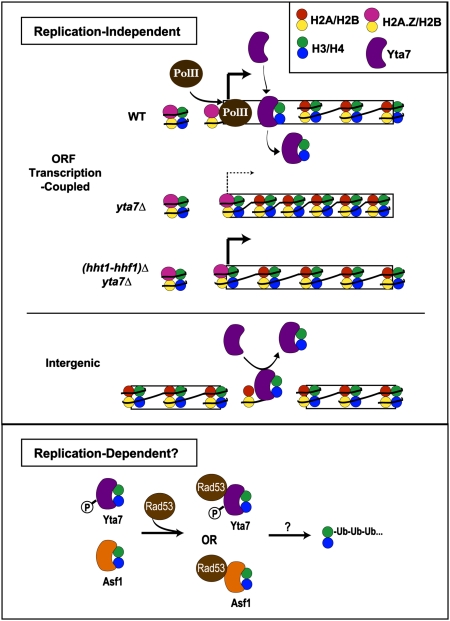
Fig. 8.
Potential mechanism of Yta7's limitation of chromatin-bound H3. Our data supported a model in which Yta7 is recruited to highly transcribed genes, where it is necessary to limit the amount and distribution of H3 occupancy, possibly functioning downstream of the histone variant H2A.Z. As depicted, transcriptional defects attributable to the increased nucleosome occupancy in cells without Yta7 were suppressed by decreasing the dosage of histones H3 and H4 (hht1-hhf1)Δ. Because Yta7 also appeared to limit H3 occupancy at intergenic regions, this may further indicate Yta7's role in replication-independent H3 exchange or suggest a modified role in replication-dependent H3 turnover. A possible mechanism of replication-dependent posttranscriptional regulation of H3, as indicated by protein association, is Yta7's collaboration with Rad53 in the H3/H4 degradation pathway. The H3/H4 chaperone, Asf1, has already been implicated in this pathway. Such modulation of Yta7 activity might occur through the purported cell-cycle-regulated post-translational modifications of Yta7. P, phosphorylation; Ub, ubiquitylation.