Table 2.
Scope of Nucleophiles in Allylic Substitution Reactions.a
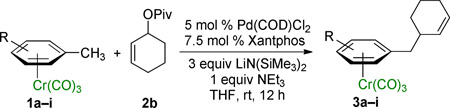 | |||
|---|---|---|---|
| Entry | Product | Compound | Yield (%) |
| 1 | 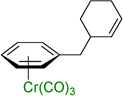 |
3a | 96b |
| 2 | 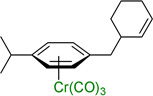 |
3b | 89b |
| 3 |  |
3c | 80b |
| 4 | 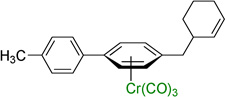 |
3d | 90b |
| 5 | 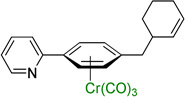 |
3e | 77b |
| 6 | 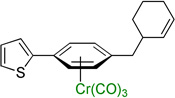 |
3f | 80b |
| 7 |  |
3g | 74b |
| 8 | 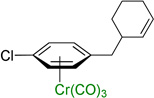 |
3h | 45b,c |
| 9 | 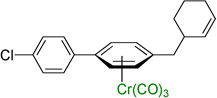 |
3i | 71b |
Reactions conducted on a 0.1 mmol scale using 1 equiv of 1 and 2 equiv of 2b at 0.1 M.
Isolated yield after chromatographic purification.
Reaction time was 1.5 h.
