Table 1.
Initial Optimization of Pd-Precatalyst and Chiral Ligand
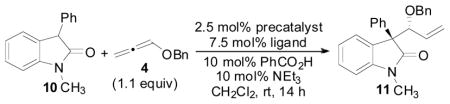 | |||||
|---|---|---|---|---|---|
| entry | precatalyst | ligand | % yielda | d.r.b | eec |
| 1 | none | none | NR | n.a. | n.a. |
| 2 | [Pd(allyl)Cl]2 | (R,R)-L1 | 68 (93) | 4.2:1 | 66 |
| 3 | [Pd(allyl)Cl]2 | (R,R)-L2 | 60 (82) | 1.4:1 | 65 |
| 4 | [Pd(allyl)Cl]2 | (R,R)-L3 | 65 (88) | 1.6:1 | 63 |
| 5 | [Pd(allyl)Cl]2 | (R,R)-L4 | 74 (88) | 1:2.7 | 15 |
| 6d | [Pd(allyl)Cl]2 | (R,R)-L1 | 75 (88) | 4.2:1 | 66 |
| 7d | Pd(OTFA)2 | (R,R)-L1 | NR | n.a. | n.a. |
| 8d | Pd(OAc)2 | (R,R)-L1 | 61 (95) | 4.6:1 | 67 |
| 9d | Pd2(dba)3-CHCl3 | (R,R)-L1 | 80 (94) | 6.2:1 | 78 |
| 10e | Pd2(dba)3-CHCl3 | (R,R)-L1 | 92 | 6.4:1 | 80 |
Determined by 1H NMR with respect to mesitylene as the internal standard, number in parantheses refers to yield based on unreacted 10.
Determined by 1H NMR of the unpurified reaction mixture.
Determined by chiral HPLC analysis.
Using 1.2 equiv 4.
Using 1.2 equiv of freshly prepared 4.
