Table 10.
Use of 3-Alkyloxindoles in the Allene Hydrocarbonation
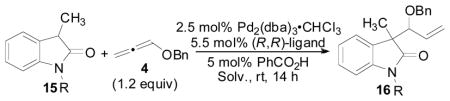 | ||||||
|---|---|---|---|---|---|---|
| entry | R | Solv. | ligand | % yielda | d.r.b | % eec |
| 1 | CH3 | THF | L1 | NR | -- | -- |
| 2 | Boc | THF | L1 | 96 | 1.4:1 | -- |
| 3 | Boc | THF | L2 | 41 (93) | 1:1.4 | -- |
| 4 | Boc | THF | L3 | 86 (>99) | 1.1:1 | -- |
| 5 | Boc | THF | L4 | 36 (99) | 1.0:1 | -- |
| 6 | Boc | MeCN | L1 | 95 | 1:1.5 | -- |
| 7 | Boc | MeCN | L4 | 92 | 1.5:1 | -- |
| 8 | CH3 | MeCN | L1 | 20 (95) | -- | -- |
| 9d | CH3 | MeCN | L1 | 53 (99) | 4.2:1 | -- |
| 10 | Bn | MeCN | L1 | 87 (98)e | 4.2:1 | 59 |
| 11d | Bn | MeCN | L1 | 84 (95) | 4.4:1 | 63 |
| 12 | Bn | MeCN | L2 | NR | -- | -- |
| 13 | Bn | MeCN | L3 | 10 (90) | -- | -- |
| 14 | Bn | MeCN | L4 | 38 (88) | 1:1.7 | -- |
Determined by 1H NMR using mesitylene as the internal standard; number in parantheses refers to yield based on unreacted 15.
Determined by 1H NMR analysis of the unpurified reaction mixture.
Determined by chiral HPLC analysis.
Reaction performed using 5 mol% Pd2 (dba)3-CHCl3 and 11 mol% (R,R)-L1.
Isolated yield was 80%.
