Table 11.
Hydrolysis of Nitrile 32
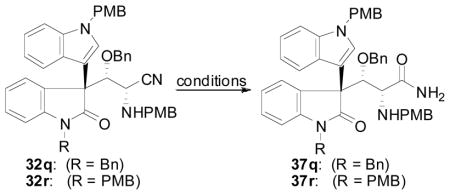 | |||
|---|---|---|---|
| entry | 32 | conditions | result |
| 1 | 32q | 26 mol% K2CO3, 10 equiv 30% H2O2, DMSO, rt | 15–75% |
| 2 | 32q | 25 mol% K2CO3, 3 equiv 30% H2O2, DMSO, rt | NR |
| 3 | 32q | 100 mol% K2CO3, 15 equiv 30% H2O2, DMSO, rt | 48% |
| 4 | 32q | 100 mol% K2CO3, 10 mol% 18-c-6, 30 equiv 30% H2O2, THF, rt | NR |
| 5 | 32q | 100 mol% K2CO3, 30 equiv 30% H2O2, TFE, rt | decomp. |
| 6 | 32q | 25 mol% K2CO3, 3 equiv 30% H2O2, MeOH, 45 °C | decomp. |
| 7 | 32q | 32 mol% Bu4NHSO4, 1 equiv 2M NaOH, 5 equiv 30% H2O2, CH2Cl2, rt | NR |
| 8 | 32q | Ba(OH)2-8H2O, DME/H2O, 65 °C | retrocyan. |
| 9 | 32q | 40 mol% K2CO3, 6 equiv urea-H2O2, DMSO, rt | 85% |
| 10 | 32r | 40 mol% K2CO3, 6 equiv urea-H2O2, DMSO, rt | 84% |
| 11 | 32q | HCl/Et2O/MeOH, rt | 45% |
| 12 | 32q | 10 mol% Parkin’s cat., EtOH/H2O (9/1), 70 °C | retrocyan. |
