Table 5.
Effects of Reaction Concentration, Catalyst, Ligand, and Acid Loadings on the Allene Hydrocarbonation Reaction
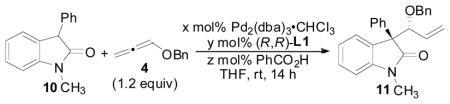 | |||||||
|---|---|---|---|---|---|---|---|
| entry | Conc.a | x | y | z | % yieldb | d.r.c | % eed |
| 1 | 0.1 M | 2.5 | 7.5 | 10 | 94 | 13:1 | 84 |
| 2e | 0.1 M | 2.5 | 7.5 | 10 | >99 | 4.5:1 | 52 |
| 3f | 0.1 M | 2.5 | 7.5 | 10 | 94 | 10:1 | 80 |
| 4 | 0.1 M | 2.5 | 7.5 | 2.5 | >99 | 11:1 | 82 |
| 5 | 0.1 M | 2.5 | 7.5 | 5 | 97 | 13:1 | 84 |
| 6 | 0.1 M | 2.5 | 7.5 | 50 | 61 (91) | 5.8:1 | 60 |
| 7 | 0.1 M | 2.5 | 7.5 | 100 | 55 (96) | 3.5:1 | −6 |
| 8 | 0.1 M | 0.5 | 1.5 | 1 | 94 | 11:1 | 82 |
| 9 | 0.1 M | 5 | 15 | 10 | >99 | 13:1 | 80 |
| 10 | 0.2 M | 2.5 | 7.5 | 5 | >99 | 10:1 | 74 |
| 11 | 0.5 M | 2.5 | 7.5 | 5 | 96 | 6.5:1 | 64 |
| 12 | 0.05 M | 2.5 | 7.5 | 5 | >99 | 14:1 | 89 |
| 13 | 0.01 M | 2.5 | 7.5 | 5 | 90 | 19:1 | 93 |
| 14 | 0.1 M | 2.5 | 6.0 | 5 | 95 | 14:1 | 87 |
| 15 | 0.1 M | 2.5 | 5.5 | 5 | 97 | 14:1 | 86 |
| 16g | 0.1 M | 2.5 | 5.5 | 5 | 95 | 11:1 | 80 |
| 17h | 0.1 M | 2.5 | 5.5 | 5 | >99 | 4.6:1 | 53 |
With respect to 10.
Determined by 1H NMR with respect to mesitylene as the internal standard; number in parantheses refers to yield based on unreacted 10.
Determined by 1H NMR analysis of the unpurified reaction mixture.
Determined by chiral HPLC analysis.
20 mol% tetrabutylammonium difluorotriphenylsilicate (TBAT) employed.
20 mol% (n-hexyl)4 NBr employed.
Reaction performed at 4 °C.
Reaction performed at −20 °C.
