Table 6.
Effect of Substituted Benzoic Acid Cocatalysts on the Allene Hydrocarbonation Reaction
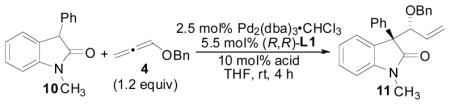 | |||||
|---|---|---|---|---|---|
| entry | acid | pKaa | % yieldb | d.r.c | % eed |
| 1 | PhCO2H | 4.22 | 95 | 13:1 | 84 |
| 2 | p-MeO-C6H4CO2H | 4.90 | 97 | 13:1 | 86 |
| 3 | p-Me-C6H4CO2H | 4.36 | 96 | 13:1 | 84 |
| 4 | p-Ph-C6H4CO2H | -- | 99 | 13:1 | 85 |
| 5 | o-MeO-C6H4CO2H | 4.20 | 93 (97) | 11:1 | 84 |
| 6 | o-Me-C6H4CO2H | 3.91 | >99 | 13:1 | 84 |
| 7 | 2-naphthoic acid | 4.17 | 99 | 15:1 | 85 |
| 8 | 1-naphthoic acid | 3.69 | >99 | 15:1 | 87 |
| 9 | p-Cl-C6H4CO2H | 3.99 | 95 | 15:1 | 84 |
| 10 | p-NO2-C6H4CO2H | 3.44 | 98 | 16:1 | 87 |
| 11 | o-Cl-C6H4CO2H | 2.92 | 90 (95) | 15:1 | 86 |
| 12 | o-NO2-C6H4CO2H | 2.17 | 87 (98) | 15:1 | 86 |
| 13 | C6F5CO2H | -- | 70 (84) | 13:1 | 81 |
Value in H2O.
Determined by 1H NMR with respect to mesitylene as the internal standard; number in parantheses refers to yield based on unreacted 10.
Determined by 1H NMR analysis of the unpurified reaction mixture.
Determined by chiral HPLC analysis.
