Table 7.
Reaction Scope Employing 3-Aryloxindoles as Nucleophile
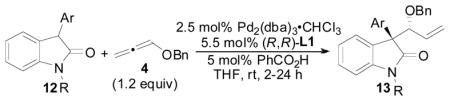 | |||||
|---|---|---|---|---|---|
| entrya | R | Ar | % yieldb | d.r.c | % eed |
| 1 | CH3 | Ph (10) | 92 | 13:1 | 87 |
| 2 | H | Ph (12a) | 70 | 1.5:1 | n.d. |
| 3 | PMB | Ph (12b) | >99 | 14:1 | 90 |
| 4 | Bn | Ph (12c) | 95 | 16:1 | 93 |
| 5 | Boc | Ph (12d) | 99 | 1:1.2 | 0 |
| 6e | Boc | Ph (12d) | 99 (92) | 1:8.0 | 0 |
| 7 | Bn | p-Ph-C6H4-(12e) | 96 (95) | 13:1 | 93 |
| 8 | Bn | p-F-C6H4-(12f) | 96 | 13:1 | 90 |
| 9 | Bn | p-Cl-C6H4-(12g) | >99 | 11:1 | 84 |
| 10 | Bn | p-Me-C6H4-(12h) | >99 (93) | 18:1 | 86 |
| 11 | Bn | p-MeO-C6H4-(12i) | 92 (84) | 17:1 | 89 |
| 12 | Bn | p-Me2N-C6H4-(12j) | 86 | 12:1 | 85 |
| 13 | Bn | m-Me-C6H4-(12k) | 92 | 17:1 | 90 |
| 14 | Bn | o-Me-C6H4-(12l) | 40 | 1:1.2 | n.d. |
| 15 | Bn | o-MeO-C6H4-(12m) | 19 | 1:2.0 | n.d. |
| 16 | Bn |
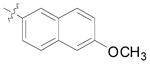 (12n) |
95 | 15:1 | 93 |
| 17 | Bn |
(12o) |
98 | 6.8:1 | 78 |
| 18f | Bn | (12o) | 90 (91) | 7.8:1 | 83 |
| 19 | Bn |
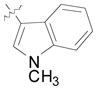 (12p) |
64 | 6.5:1 | 84 |
| 20g,h | Bn | (12p) | (94) | 7.0:1 | 88 |
| 21g,i,j | Bn |
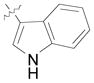 (12q) |
(71) | 7.7:1 | 91 |
| 22g,i,k | PMB |
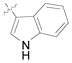 (12r) |
(80) | 7.4:1 | 91 |
0.2 mmol scale (0.1 M) relative to 12 at rt for 2 to 24 h.
Determined by 1H NMR with respect to mesitylene as the internal standard; number in parantheses refers to isolated yield.
Determined by 1H NMR analysis of the unpurified reaction mixture.
Determined by chiral HPLC analysis.
Ligand L4 was used.
p-nitrobenzoic acid was used in place of PhCO2H.
1-naphthoic acid was used in place of PhCO2H.
1.56 mmol scale.
7.5 mol% of (R,R)-L1 was used.
1.73 mmol scale.
2.71 mmol scale.
