Table 8.
Reaction Optimization Employing ortho-Substituted Aryl Rings
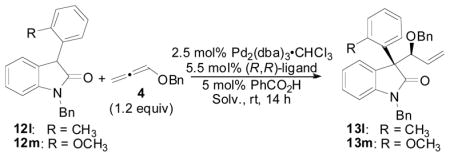 | ||||||
|---|---|---|---|---|---|---|
| Entrya | 12 | ligand | Solv. | % yieldb | d.r.c | % eed |
| 1 | 12l | L1 | THF | 40 | 1:1.2 | -- |
| 2 | 12l | L1 | CH2Cl2 | 42 | 2:1 | -- |
| 3 | 12l | L1 | DMF | 75 | 11:1 | 90 |
| 4 | 12l | L1 | MeCN | >99 (93) | 13:1 | 95 |
| 5 | 12m | L1 | THF | 19 | 1:2 | 11 |
| 6 | 12m | L1 | MeCN | 95 (84) | 10:1 | 95 |
| 7 | 12m | L2 | MeCN | 10 | 2.4:1 | -- |
| 8 | 12m | L3 | MeCN | 84 | 2.5:1 | 65 |
| 9 | 12m | L4 | MeCN | 46 | 1.7:1 | −35 |
Reactions were performed on a 0.2 mmol scale (0.1 M) relative to 12. The absolute stereochemistry of 13l and 13m was not determined
Determined by 1H NMR; number in parantheses refers to isolated yield.
Determined by 1H NMR analysis of the unpurified reaction mixture.
Determined by chiral HPLC analysis.
