Table 9.
Scope of the Allene Coupling Partner
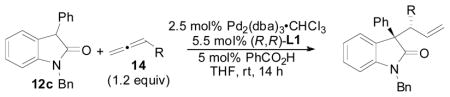 | ||||
|---|---|---|---|---|
| entrya | allene | % yieldb | d.r.c | % eed |
| 1 |
|
>99 (96) | 17:1 | 87 |
| 2 |
|
>99 | 8.0:1 | 78 |
| 3 |
|
>99 | 2.7:1 | 27 |
| 4 |
|
NR | -- | -- |
| 5 |
|
NR | -- | -- |
| 6 |
|
NR | -- | -- |
| 7e |
|
(62) | 6.5:1 | 86 |
Reactions were performed on a 0.2 mmol scale (0.1 M) relative to 12c.
Determined by 1H NMR with respect to mesitylene as the internal standard; number in parantheses refers to isolated yield.
Determined by 1H NMR analysis of the unpurified reaction mixture.
Determined by chiral HPLC analysis.
Reaction performed using oxindole 12q using 7.5 mol% (R,R)-L1 and 5 mol% 1-naphthoic acid at rt for 63 h.
