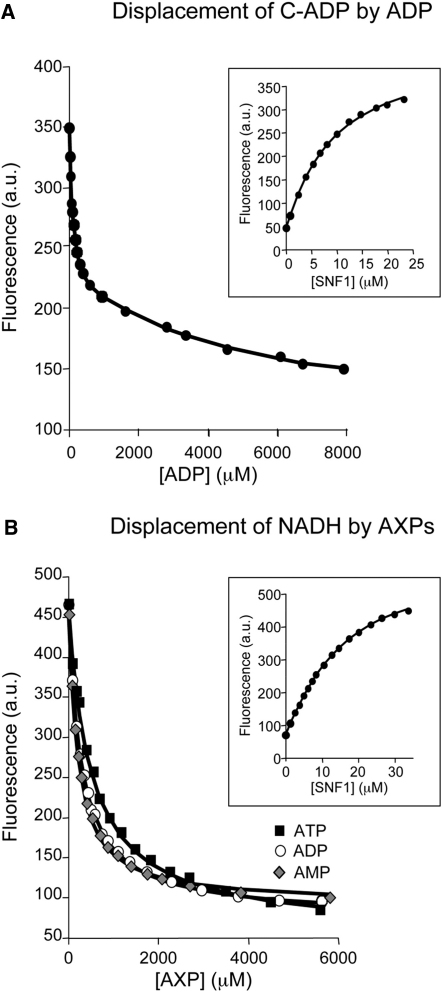
Figure 2.
Measurement of Equilibrium Dissociation Constants for the Binding of AXPs to Phosphorylated SNF1
(A) Displacement of coumarin-ADP (C-ADP) from the SNF1:(C-ADP)2 complex by AXPs monitored using the change in fluorescence at 470 nm (excitation at 430 nm). The solid lines are the computed best fits with Kd,I and Kd,II for C-ADP binding fixed at 9 and 27 μM, respectively. Inset: titration of C-ADP with SNF1.
(B) Displacement of NADH from the SNF1:NADH complex by AXPs monitored using the change in fluorescence at 435 nm (excitation at 340 nm). The solid lines are the computed best fits with the Kd for NADH fixed at 12.3 μM Inset: titration of NADH with SNF1. All measurements were carried out at 20°C in 50 mM Tris (pH 8), 100 mM NaCl, 1 mM tris(2-carboxyethyl)phosphine.