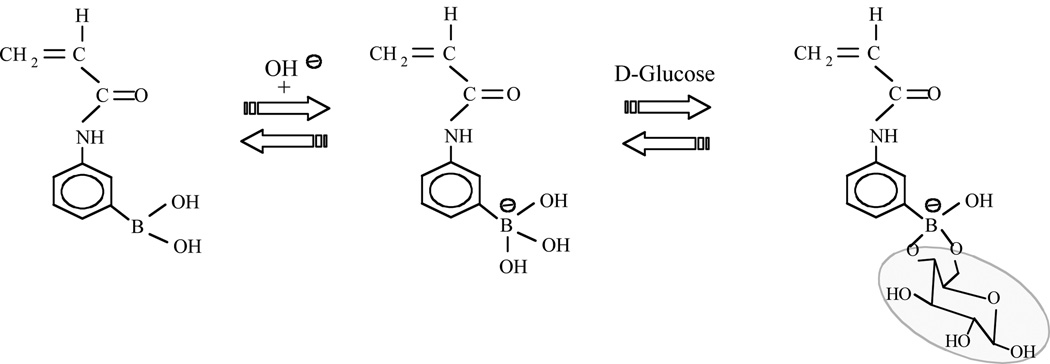
Figure 1.
From left-to-right, the interaction between the boronic acid moiety (on acrylamide monomer) and a hydroxyl group to form the charged boronate moiety; reversible binding between the boronate moiety and a diol such as on D-glucose to form a cyclic boronic acid ester (tridentate binding is also possible).