Abstract
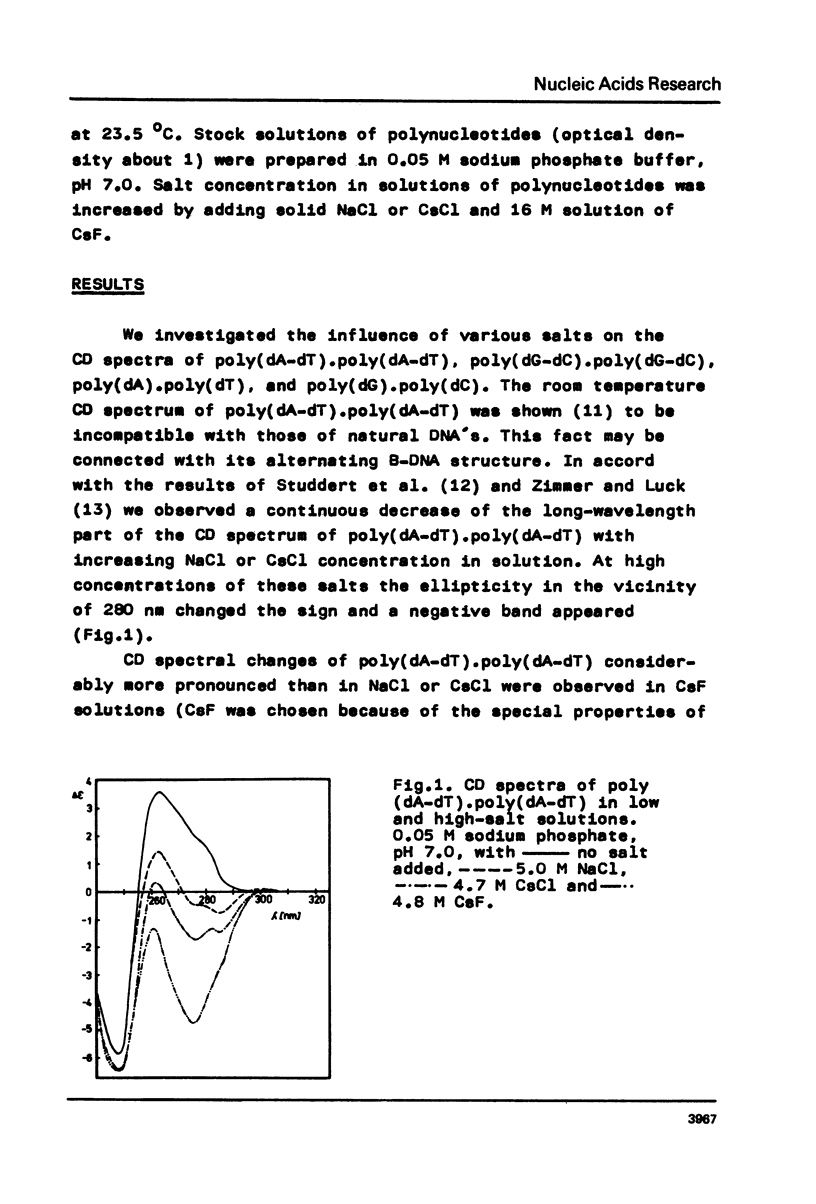
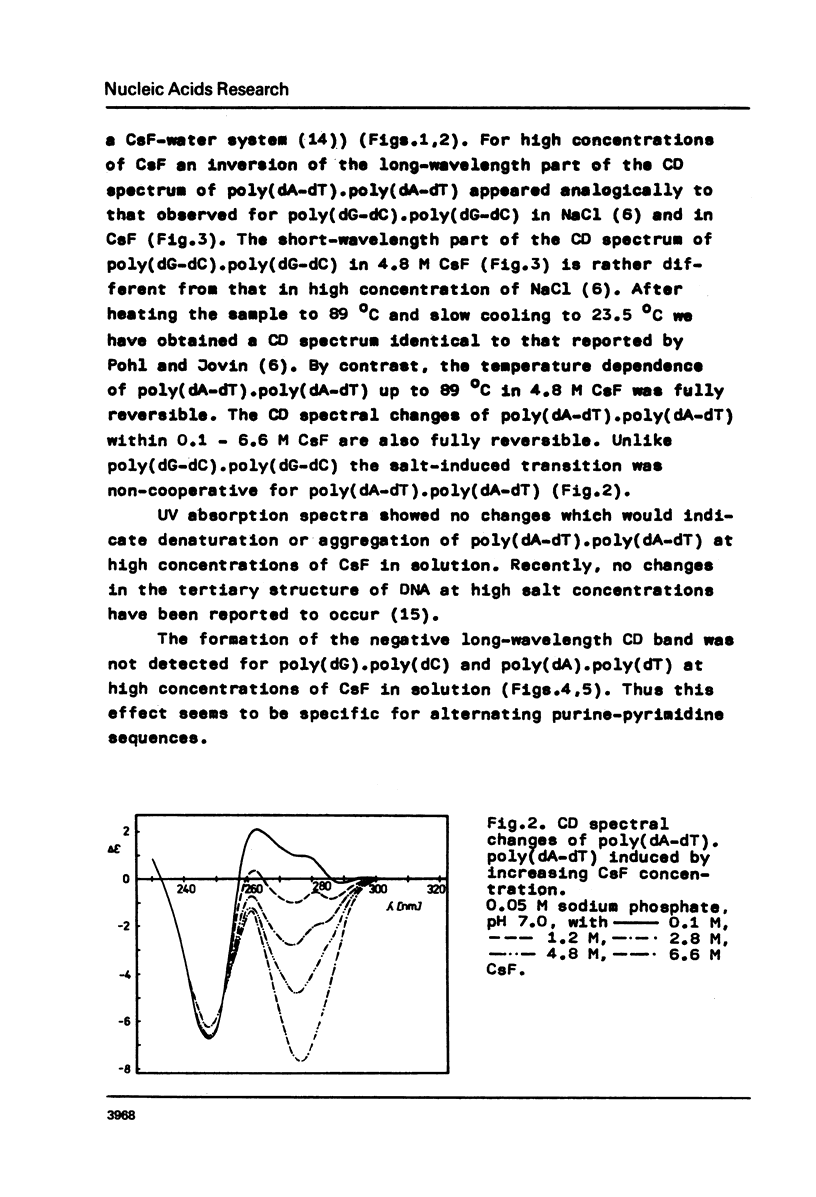
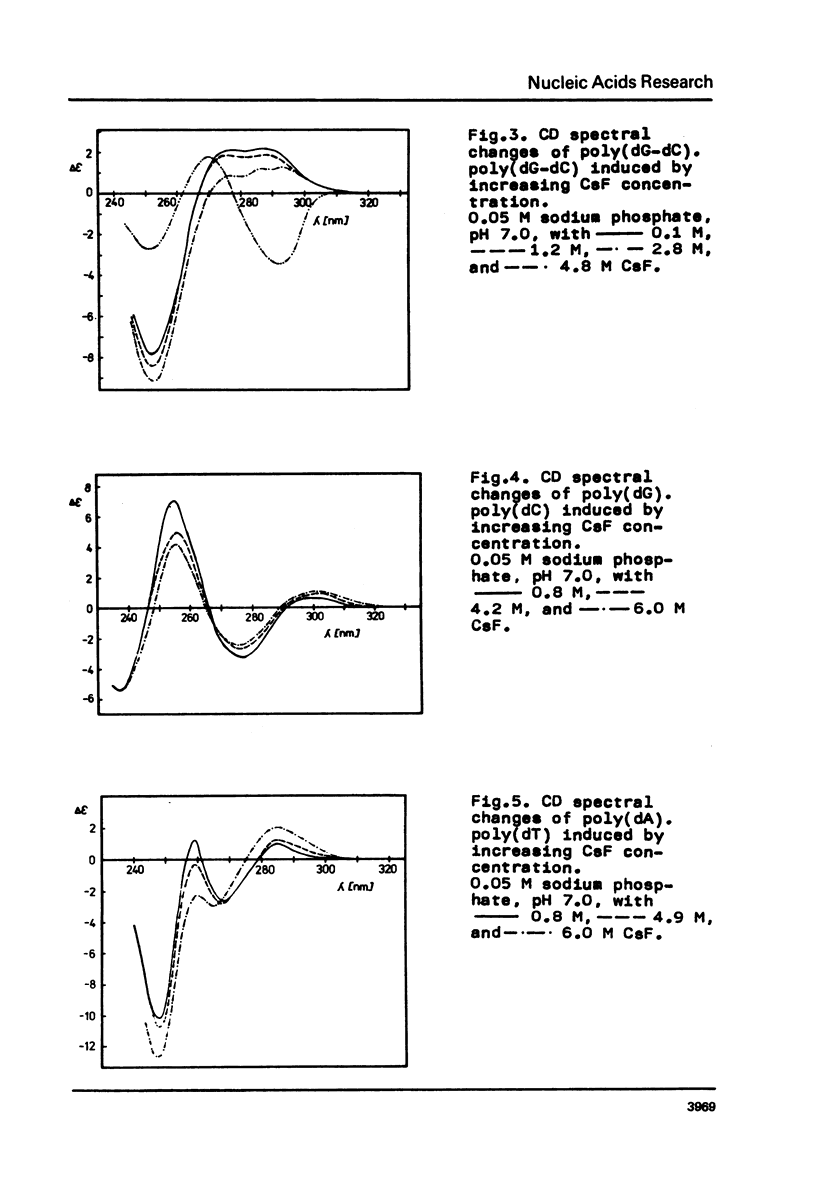
Conformational changes of poly(dA-dT) . poly(dA-dT) induced by increasing ionic strength were studied using CD spectroscopy. It was found that a pronounced noncooperative inversion of the long-wavelength part of the CD spectrum of poly(dA-dT) . poly(dA-dT) occurred at high concentrations of CsF in solution. It was suggested that a great difference between the geometries of the purine and pyrimidine residues in the helix was characteristic of the structure of poly(dA-dT) . poly(dA-dT) in concentrated CsF solutions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Burckhardt G., Zimmer Ch, Luck G. Conformation and reactivity of DNA V. pH-dependent conformational changes of DNA in complexes with poly-L-histidine: Transitions from B- to A-form and to a condensed state. FEBS Lett. 1973 Feb 15;30(1):35–39. doi: 10.1016/0014-5793(73)80613-1. [DOI] [PubMed] [Google Scholar]
- Evdokimov Y. M., Pyatigorskaya T. L., Polyvtsev O. F., Akimenko N. M., Kadykov V. A., Tsvankin D. Y., Varshavsky Y. M. A comparative X-ray diffraction and circular dichroism study of DNA compact particles formed in water-salt solutions, containing poly(ethylene glycol). Nucleic Acids Res. 1976 Sep;3(9):2353–2366. doi: 10.1093/nar/3.9.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasman G. D., Schaffhausen B., Goldsmith L., Adler A. Conformational changes associated with f-1 histone-deoxyribonucleic acid complexes. Circular dichroism studies. Biochemistry. 1970 Jul 7;9(14):2814–2822. doi: 10.1021/bi00816a010. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Findlay J. B., Momii R. K., Luxon B. A., Kar D. Temperature dependence of the 31P chemical shifts of nucleic acids. A prode of phosphate ester torsional conformations. Biochemistry. 1976 Aug 24;15(17):3796–3803. doi: 10.1021/bi00662a023. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Jordan C. F., Lerman L. S., Venable J. H. Structure and circular dichroism of DNA in concentrated polymer solutions. Nat New Biol. 1972 Mar 22;236(64):67–70. doi: 10.1038/newbio236067a0. [DOI] [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Lezius A. G., Gottschalk E. M. Uber eine reversible kooperative Konformationsumwandlung einer synthetischern DNA unter dem Einfluss hoher Salzkonzentrationen. Hoppe Seylers Z Physiol Chem. 1970 Mar;351(3):413–416. [PubMed] [Google Scholar]
- Mitsui Y., Langridge R., Shortle B. E., Cantor C. R., Grant R. C., Kodama M., Wells R. D. Physical and enzymatic studies on poly d(I-C)-poly d(I-C), an unusual double-helical DNA. Nature. 1970 Dec 19;228(5277):1166–1169. doi: 10.1038/2281166a0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Sarocchi M. T., Guschlbauer W. Protonated polynucleotide structures. Sequence-dependent and protonation-sensitive metastable states in DNA premelting. Eur J Biochem. 1973 Apr;34(2):232–240. doi: 10.1111/j.1432-1033.1973.tb02751.x. [DOI] [PubMed] [Google Scholar]
- Shindo H., Simpson R. T., Cohen J. S. An alternating conformation characterizes the phosphodiester backbone of poly(dA-dT) in solution. J Biol Chem. 1979 Sep 10;254(17):8125–8128. [PubMed] [Google Scholar]
- Sponar J., Fric I. Complexes of histone F1 with DNA in 0.15M NaCl. Circular dichroism and structure of the complexes. Biopolymers. 1972;11(11):2317–2330. doi: 10.1002/bip.1972.360111110. [DOI] [PubMed] [Google Scholar]
- Studdert D. S., Patroni M., Davis R. C. Circular dichroism of DNA: temperature and salt dependence. Biopolymers. 1972;11(4):761–779. doi: 10.1002/bip.1972.360110404. [DOI] [PubMed] [Google Scholar]
- Thomas J. C., Schurr J. M. Dynamic light-scattering studies of DNA. II. Effect of ionic strength on the structure and internal dynamics of viral phi 29 DNA. Biopolymers. 1980 Jan;19(1):215–218. doi: 10.1002/bip.1980.360190115. [DOI] [PubMed] [Google Scholar]
- Tunis-Schneider M. J., Maestre M. F. Circular dichroism spectra of oriented and unoriented deoxyribonucleic acid films--a preliminary study. J Mol Biol. 1970 Sep 28;52(3):521–541. doi: 10.1016/0022-2836(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wolf B., Berman S., Hanlon S. Structural transitions of calf thymus DNA in concentrated LiCl solutions. Biochemistry. 1977 Aug 9;16(16):3655–3662. doi: 10.1021/bi00635a023. [DOI] [PubMed] [Google Scholar]
- Zama M., Ichimura S. Difference between polylysine and polyarginine in changing DNA structure upon complex formation. Biochem Biophys Res Commun. 1971 Aug 20;44(4):936–942. doi: 10.1016/0006-291x(71)90802-3. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G. Conformation and reactivity of DNA. VI. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim Biophys Acta. 1974 Aug 15;361(1):11–32. [PubMed] [Google Scholar]



