Abstract
BACKGROUND
Blood-stage malaria vaccines are intended to prevent clinical disease. The malaria vaccine FMP2.1/AS02A, a recombinant protein based on apical membrane antigen 1 (AMA1) from the 3D7 strain of Plasmodium falciparum, has previously been shown to have immunogenicity and acceptable safety in Malian adults and children.
METHODS
In a double-blind, randomized trial, we immunized 400 Malian children with either the malaria vaccine or a control (rabies) vaccine and followed them for 6 months. The primary end point was clinical malaria, defined as fever and at least 2500 parasites per cubic millimeter of blood. A secondary end point was clinical malaria caused by parasites with the AMA1 DNA sequence found in the vaccine strain.
RESULTS
The cumulative incidence of the primary end point was 48.4% in the malaria-vaccine group and 54.4% in the control group; efficacy against the primary end point was 17.4% (hazard ratio for the primary end point, 0.83; 95% confidence interval [CI], 0.63 to 1.09; P = 0.18). Efficacy against the first and subsequent episodes of clinical malaria, as defined on the basis of various parasite-density thresholds, was approximately 20%. Efficacy against clinical malaria caused by parasites with AMA1 corresponding to that of the vaccine strain was 64.3% (hazard ratio, 0.36; 95% CI, 0.08 to 0.86; P = 0.03). Local reactions and fever after vaccination were more frequent with the malaria vaccine.
CONCLUSIONS
On the basis of the primary end point, the malaria vaccine did not provide significant protection against clinical malaria, but on the basis of secondary results, it may have strain-specific efficacy. If this finding is confirmed, AMA1 might be useful in a multicomponent malaria vaccine.
An effective malaria vaccine would improve the prospects for eradicating malaria.1 Vaccines that interrupt the transmission of malaria are emphasized in discussions of eradication,2 but the ideal malaria vaccine would provide a direct clinical benefit. Vaccines targeting the blood stages of malaria are intended to reduce morbidity and mortality and are being developed in hopes of creating a multistage, multi-antigen vaccine.3
Vaccines based on two polymorphic Plasmodium falciparum blood-stage proteins, merozoite surface protein 14 and apical membrane antigen 1 (AMA1),5 were not shown to be effective in recent studies, probably because of insufficient cross-protection against diverse malaria strains6,7 or insufficient immunogenicity. A vaccine based on three non-AMA1 blood-stage antigens failed to prevent clinical malaria but reduced the risk of infections with vaccine-type strains.8 No such strain specificity was seen with a vaccine based on a pre-erythrocytic protein that prevents clinical malaria.9–11
FMP2.1/AS02A is a monovalent blood-stage malaria vaccine based on AMA1 from the 3D7 strain of P. falciparum.12 It had acceptable safety and tolerability in volunteers in the United States with no history of malaria13,14 and in malaria-exposed adults15 and children16 in Mali. In all these populations, FMP2.1/AS02A produced higher and more sustained antibody responses than a bivalent AMA1 vaccine that showed neither overall5 nor strain-specific17 efficacy in a recent trial in Mali. We conducted a proof-of-concept trial to assess the efficacy of FMP2.1/AS02A against clinical falciparum malaria in Malian children and to determine whether the efficacy was strain-specific.
METHODS
STUDY DESIGN
We conducted a randomized, controlled, double-blind trial. The protocol (available with the full text of this article at NEJM.org) was approved by the institutional review boards at the University of Bamako Faculty of Medicine, the University of Maryland, Baltimore, the Walter Reed Army Institute of Research, and the U.S. Army Surgeon General. The National Institute of Allergy and Infectious Diseases and the U.S. Agency for International Development reviewed the study protocol. The trial was monitored by the National Institute of Allergy and Infectious Diseases and the U.S. Army Medical Material Development Activity. An independent data and safety monitoring board and a local safety monitor were appointed by the National Institute of Allergy and Infectious Diseases. The trial was conducted in compliance with the study protocol, the International Conference on Harmonization of Good Clinical Practices, the Declaration of Helsinki, and regulatory requirements of Mali. Program staff from the National Institute of Allergy and Infectious Diseases contributed to the study design but played no role in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit it for publication, all of which were done by the authors. Authors from GlaxoSmithKline Biologicals contributed to the study design, provided edits to the version of the manuscript submitted for publication (including interpretation of the data), and agreed with the decision to submit the manuscript for publication; these industry authors played no role in the collection or analysis of the data. Data management and statistical support were provided by EMMES, a contract research organization. All authors vouch for the veracity and completeness of the data presented as well as the fidelity of this report to the study protocol.
STUDY PARTICIPANTS
The study was conducted at the Bandiagara Malaria Project research clinic in rural Mali from May 2007 through February 2008. Four hundred healthy children 1 to 6 years of age were enrolled and randomly assigned to receive either the malaria vaccine or a rabies vaccine (control vaccine). Written informed consent was obtained from a parent or guardian of each child. Active and passive surveillance for malaria began at the time of the first vaccination and continued throughout the follow-up period. Venous-blood samples were obtained for safety and immunogenicity analyses on the day of each vaccination and 1, 3, and 6 months after the third vaccination; samples were also collected 1 week after each vaccination for the safety analysis. The duration of follow-up for the primary analysis was 8 months (i.e., the 2-month vaccination period and 6 months of postvaccination surveillance, spanning one malaria season).
VACCINES
Children received either 50 μg of lyophilized FMP2.1 (from the Walter Reed Army Institute of Research), which was resuspended shortly before vaccination in 0.5 ml of adjuvant (AS02A, Glaxo-SmithKline Biologicals),15 or 1 ml of purified chick-embryo rabies vaccine (RabAvert, Chiron Vaccines). Vaccines were prepared in identical syringes and covered to conceal their contents.
RANDOMIZATION AND VACCINATION
Children were randomly assigned, in a 1:1 ratio, to receive three doses (one dose each month) of either vaccine. Block randomization was used, with stratification by age group. Participants were assigned study numbers in the order in which they arrived at the clinic on the first day of vaccination. Vaccines were administered intramuscularly in the left deltoid.
EFFICACY END POINTS
All primary, secondary, and exploratory end points were specified in the study protocol and in a statistical analysis plan that was finalized before unblinding. The primary end point was a clinical episode of malaria, defined as fever (axillary temperature of 37.5°C or higher) with an asexual P. falciparum density of at least 2500 parasites per cubic millimeter of blood. Secondary end points included one or more episodes of clinical malaria due to parasites that had AMA1 genotypes identical to the 3D7 vaccine strain with respect to designated immunologically important AMA1 polymorphisms (codons 196, 197, 199, 200, 201, 204, 206, and 207 in the cluster 1 loop of domain I)7 and the incidence of multiple episodes of clinical malaria. Exploratory efficacy end points included clinical episodes of malaria according to various definitions (i.e., parasitemia thresholds ranging from any level above 0 to 100,000 parasites per cubic millimeter and episodes with fever or with symptoms consistent with malaria, irrespective of the axillary temperature), cumulative asexual P. falciparum parasite density measured for each child as the total area under the curve (AUC) for parasitemia in both clinical malaria episodes and asymptomatic infections detected in monthly surveys, and the incidence of anemia, defined (on the basis of local norms) as a hemoglobin level of less than 8.4 g per deciliter.
MONITORING FOR EFFICACY AND SAFETY
Passive case detection was achieved by means of continuously available medical care, including prompt diagnosis and treatment of malaria. Active surveillance to detect asymptomatic malaria and anemia consisted of frequent, scheduled clinic visits during the vaccination period, followed by monthly visits during the 6-month period after the third vaccination. Blood smears collected at monthly visits were not read at the time of collection unless symptoms were present. Solicited adverse events were recorded on vaccination days and 1, 2, 3, and 7 days after each vaccination. Unsolicited adverse events (those reported spontaneously by a parent or guardian) were recorded throughout the 8-month study period.
PARASITE GENOTYPING
DNA was extracted from dried-blood spots collected during clinical malaria episodes, and the gene encoding P. falciparum AMA1 was sequenced.7 Clinical episodes were classified according to whether the AMA1 sequence of the infecting parasites matched or did not match that of the vaccine strain 3D7, with matching ascertained as complete concordance at eight polymorphic amino acid positions in the cluster 1 loop of AMA1 domain I. These eight positions had been identified before the analysis as important in the development of strain-specific immunity against AMA1, on the basis of both in vitro18 and molecular epidemiologic7 evidence.
STATISTICAL ANALYSIS
The study was designed to have 90% power to detect an efficacy rate of 20% for the malaria vaccine, with a two-tailed significance level of 0.05, assuming a 75% incidence of a first episode of clinical malaria. This relatively low overall efficacy rate was chosen in anticipation that the vaccine might have strain-specific efficacy only. All reported P values are two-sided.
The primary analysis estimated the hazard ratio for the first or only episode of clinical malaria in the intention-to-treat cohort (all randomly assigned children, with data collection starting on the day of the first vaccination). Secondary efficacy analyses were conducted to estimate the hazard ratio for the first or only clinical episode of malaria (according to the definition of the primary end point) with an AMA1 genotype matching that of the vaccine strain,7 the hazard ratio for multiple episodes, and the same end points in the per-protocol cohort (children who received all three doses of the assigned vaccine and completed at least 14 days of follow-up after the third vaccination, with data collection starting 14 days after the third vaccination).
The relationship between anti-AMA1 antibodies and the risk of clinical malaria was examined by estimating the hazard ratio for the first or only episode of clinical malaria. To estimate vaccine-induced (as opposed to naturally acquired) anti-AMA1 antibodies, we subtracted the log10-transformed antibody level at baseline from the level at the time point, at least 2 weeks after the third vaccination, that was closest to but not after the first malaria episode.
Cumulative parasite density was estimated for each child by measuring the AUC for the entire study period and also for the period from 2 weeks after the third vaccination until the end of the follow-up period. All recorded episodes of parasitemia were included, irrespective of the presence or absence of symptoms of malaria; parasite density was assumed to decline linearly to zero 3 days after a treated malaria episode. Additional details of the methods are given in the Supplementary Appendix, available at NEJM.org.
RESULTS
PARTICIPANTS
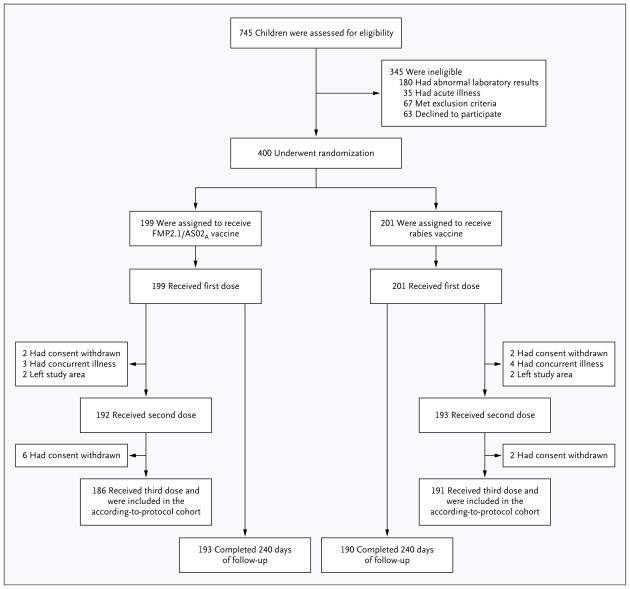
A total of 745 children were screened; 400 were randomly assigned to a vaccine, received at least one vaccination, and were included in the intention-to-treat analysis. A total of 186 of 199 children (93.5%) in the malaria-vaccine group and 191 of 201 (95.0%) in the control group received all three immunizations. Of all 400 children, 383 (95.8%) completed follow-up to study day 240 (Fig. 1). Demographic and other characteristics were balanced between the two groups at enrollment (Table 1).
Figure 1.
Enrollment and Follow-up of the Study Participants.
Table 1.
Baseline Characteristics of the Study Participants, According to Vaccine Group.*
| Characteristic | FMP2.1/AS02A Vaccine (N = 199) | Rabies Vaccine (N = 201) | P Value |
|---|---|---|---|
| Age — yr | 3.4±1.6 | 3.4±1.5 | 0.94 |
| Female sex — no. (%) | 103 (51.8) | 112 (55.7) | 0.43 |
| White-cell count — ×10−3/mm3 | 8.95±2.50 | 8.88±2.46 | 0.78 |
| Hemoglobin — g/dl | 10.8±0.9 | 10.7±0.9 | 0.23 |
| Platelet count — ×10−3/mm3 | 340±110 | 342±106 | 0.83 |
| Lymphocyte count — ×10−3/mm3 | 4.93±1.85 | 4.96±1.81 | 0.90 |
| Creatinine — μmol/liter† | 44.2±0.5 | 44.3±0.9 | 0.66 |
| Alanine aminotransferase — U/liter | 15.08±9.28 | 15.98±16.77 | 0.80 |
| Anti–AMA1 antibody — g/ml | — | ||
| Geometric mean titer | 0.73 | 0.85 | |
| 95% CI | 0.51–1.05 | 0.59–1.22 | |
| Used an insecticide–treated bed net the previous night — no. (%) | 62 (31.2) | 61 (30.3) | 0.94 |
Plus–minus values are means ±SD. AMA1 denotes apical membrane antigen 1, and CI confidence interval.
The lower limit of detection for creatinine was 44.2 μmol per liter (0.5 mg/dl); results below the limit of detection were recorded as 44.2 μmol per liter. To convert values for creatinine to milligrams per deciliter, divide by 88.4.
EFFICACY
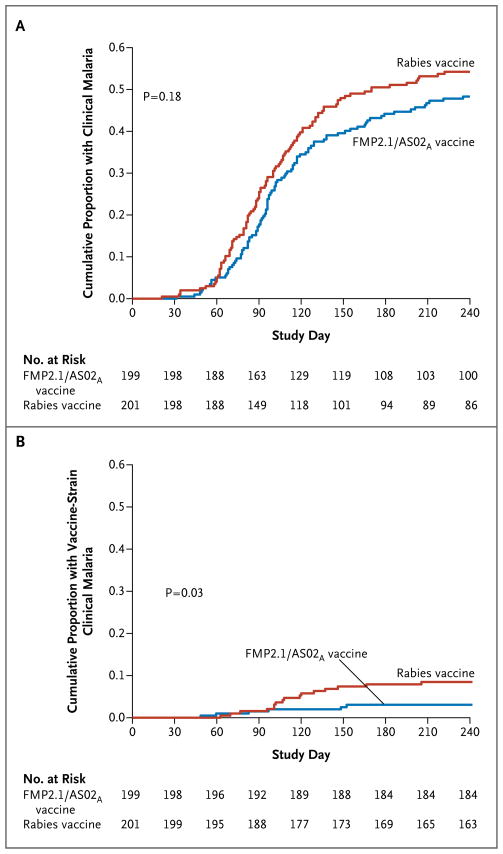
The Kaplan–Meier cumulative incidence of the first or only malaria episode, as defined for the primary end point, was 48.4% in the malaria-vaccine group and 54.4% in the control group. The unadjusted efficacy of the malaria vaccine as compared with the control vaccine, ascertained by means of Cox regression analysis, was 17.4% (hazard ratio for the primary end point, 0.83; 95% confidence interval [CI], 0.63 to 1.09; P=0.18) (Fig. 2A). Of 22 clinical episodes of infection with a P. falciparum strain with vaccine-type AMA1, 16 occurred in the control group; efficacy against clinical malaria with vaccine-type AMA1 was 64.3% (hazard ratio with malaria vaccine vs. control vaccine, 0.36; 95% CI, 0.08 to 0.86; P = 0.03) (Fig. 2B). Efficacy against multiple clinical episodes as defined for the primary end point was 20.0% (hazard ratio, 0.80; 95% CI, 0.63 to 1.02; P = 0.07) and remained approximately 20% with the use of various definitions of malaria; the more permissive definitions (i.e., those that resulted in an increased number of identified episodes) were associated with significant between-group differences (P<0.05) (Table 2). Per-protocol analyses produced similar efficacy estimates (see the Supplementary Appendix).
Figure 2. Kaplan–Meier Estimates of the Cumulative Proportion of Children in the Intention-to-Treat Cohort with at Least One Episode of Clinical Malaria over Time, According to Vaccine Group.
Clinical malaria was defined as a fever with a parasite density of at least 2500 parasites per cubic millimeter. Panel A shows the data for all (first or only) clinical malaria episodes. Panel B shows the data for all (first or only) clinical malaria episodes involving infection with a Plasmodium falciparum strain with an apical membrane antigen 1 (AMA1) sequence homologous to that of the vaccine strain. Immunizations were administered on study days 0, 30, and 60.
Table 2.
Vaccine Efficacy against First and Multiple Episodes of Clinical Malaria in the Intention-to-Treat Cohort.
| Definition of Clinical Malaria | First Episode | Multiple Episodes | ||||||
|---|---|---|---|---|---|---|---|---|
| FMP2.1/ AS02A Vaccine | Rabies Vaccine | Percent Efficacy (95% CI) | P Value | FMP2.1/ AS02A Vaccine | Rabies Vaccine | Percent Efficacy (95% CI) | P Value | |
| no. of children | no. of episodes | |||||||
| Any axillary temperature
| ||||||||
| Parasite load, >0/mm3 | 117 | 134 | 22.1 (0.2 to 39.2) | 0.048 | 176 | 224 | 22.2 (5.2 to 36.1) | 0.01 |
|
| ||||||||
| Parasite load, ≥100/mm3 | 116 | 133 | 22.3 (0.4 to 39.5) | 0.047 | 173 | 222 | 22.8 (5.9 to 36.8) | 0.01 |
|
| ||||||||
| Parasite load, ≥1000/mm3 | 110 | 126 | 22.6 (0.0 to 40.0) | 0.05 | 155 | 204 | 24.8 (7.3 to 38.9) | 0.008 |
|
| ||||||||
| Parasite load, ≥2500/mm3 | 107 | 123 | 22.1 (−0.9 to 39.9) | 0.06 | 149 | 193 | 23.5 (5.3 to 38.2) | 0.01 |
|
| ||||||||
| Parasite load, ≥5000/mm3 | 106 | 122 | 22.0 (−1.2 to 39.9) | 0.06 | 146 | 188 | 23.1 (4.5 to 38.0) | 0.02 |
|
| ||||||||
| Parasite load, ≥10,000/mm3 | 97 | 115 | 23.9 (0.3 to 41.9) | 0.048 | 129 | 175 | 27.0 (8.3 to 41.8) | 0.007 |
|
| ||||||||
| Parasite load, ≥20,000/mm3 | 86 | 104 | 23.6 (−1.7 to 42.6) | 0.07 | 112 | 147 | 24.5 (3.4 to 40.9) | 0.03 |
|
| ||||||||
| Parasite load, ≥50,000/mm3 | 61 | 71 | 16.7 (−17.3 to 40.8) | 0.30 | 72 | 91 | 21.5 (−7.0 to 42.4) | 0.13 |
|
| ||||||||
| Parasite load, ≥100,000/mm3 | 29 | 36 | 20.4 (−29.8 to 51.2) | 0.36 | 32 | 41 | 22.5 (−23.1 to 51.2) | 0.28 |
|
| ||||||||
| Axillary temperature ≥37.5°C
| ||||||||
| Parasite load, >0/mm3 | 105 | 113 | 12.7 (−13.8 to 33.1) | 0.32 | 144 | 167 | 14.5 (−6.9 to 31.6) | 0.17 |
|
| ||||||||
| Parasite load, ≥100/mm3 | 103 | 112 | 14.0 (−12.4 to 34.2) | 0.27 | 141 | 166 | 15.8 (−5.5 to 32.7) | 0.14 |
|
| ||||||||
| Parasite load, ≥1000/mm3 | 98 | 108 | 16.6 (−9.7 to 36.6) | 0.19 | 126 | 155 | 19.4 (−2.0 to 36.3) | 0.07 |
|
| ||||||||
| Parasite load, ≥2500/mm3 (primary end point) | 95 | 106 | 17.4 (−8.9 to 37.4) | 0.18 | 121 | 150 | 20.0 (−1.6 to 37.1) | 0.07 |
|
| ||||||||
| Parasite load, ≥5000/mm3 | 94 | 105 | 17.8 (−8.6 to 37.8) | 0.17 | 119 | 149 | 20.8 (−0.8 to 37.8) | 0.06 |
|
| ||||||||
| Parasite load, ≥10,000/mm3 | 86 | 100 | 20.9 (−5.6 to 40.7) | 0.11 | 108 | 139 | 23.0 (1.0 to 40.1) | 0.04 |
|
| ||||||||
| Parasite load, ≥20,000/mm3 | 78 | 88 | 16.6 (−13.2 to 38.5) | 0.25 | 94 | 116 | 19.6 (−5.6 to 38.7) | 0.12 |
|
| ||||||||
| Parasite load, ≥50,000/mm3 | 54 | 59 | 11.7 (−27.7 to 39.0) | 0.51 | 61 | 74 | 18.2 (−14.9 to 41.7) | 0.25 |
|
| ||||||||
| Parasite load, ≥100,000/mm3 | 26 | 29 | 11.4 (−50.4 to 47.8) | 0.65 | 28 | 34 | 18.2 (−34.9 to 50.4) | 0.43 |
The median individual cumulative AUC for parasite density was 168,600 parasites per cubic millimeter in the vaccine group and 376,900 in the control group in the intention-to-treat analysis and 97,700 per cubic millimeter in the malaria-vaccine group and 308,600 in the control group in the per-protocol analysis (P = 0.01 for both analyses). Figure 2 in the Supplementary Appendix shows cumulative total AUC for parasitemia in each group.
There were 17 anemia episodes in 16 children in the vaccine group and 18 episodes in 16 children in the control group (P = 1.00). No significant differences between the two groups were seen in the incidence of anemia during clinical malaria episodes or in the incidence of severe anemia, both of which were rare.
SAFETY AND REACTOGENICITY
Results for safety and reactogenicity were similar to those in earlier trials of this vaccine in the same population.15,16 There were no deaths and no vaccine-related serious adverse events. Serious adverse events occurred in five children in the malaria-vaccine group (one episode each of severe malaria, febrile seizure associated with malaria, respiratory distress attributed to bronchiolitis, severe dehydration and malnutrition, and paralytic ileus) and in one child in the control group (febrile seizure associated with malaria). Laboratory safety tests revealed no significant differences between the two groups in the number of out-of-range values or of severity values of grade 2 or higher (Table 3).
Table 3.
Unsolicited and Laboratory Adverse Events in the Intention-to-Treat Cohort, According to Vaccine Group.
| Adverse Event | FMP2.1/AS02A Vaccine | Rabies Vaccine | P Value* | ||
|---|---|---|---|---|---|
| No. of Children (N = 199) | Percent (95% CI) | No. of Children (N = 201) | Percent (95% CI) | ||
|
Unsolicited adverse event†
| |||||
| Any | 198 | 99.5 (98.5 to 100.5) | 195 | 97.0 (94.7 to 99.4) | 0.12 |
|
| |||||
| Fever | 80 | 40.2 (33.4 to 47.0) | 35 | 17.4 (12.2 to 23.7) | <0.001 |
|
| |||||
| Gastrointestinal disorder | 66 | 33.2 (26.6 to 39.7) | 74 | 36.8 (30.1 to 43.5) | 0.46 |
|
| |||||
| Respiratory disorder | 94 | 47.2 (40.3 to 54.2) | 99 | 49.3 (42.3 to 56.2) | 0.69 |
|
| |||||
| Eye disorder | 31 | 15.6 (10.5 to 20.6) | 30 | 14.9 (10.0 to 19.9) | 0.89 |
|
| |||||
| Skin or subcutaneous-tissue disorder | 29 | 14.6 (9.7 to 19.5) | 22 | 10.9 (6.6 to 15.3) | 0.30 |
|
| |||||
| Severity grade 3 (prevents everyday activities) | 3 | 1.5 (−0.2 to 3.2) | 2 | 1.0 (−0.4 to 2.4) | 0.68 |
|
| |||||
| Serious | 5 | 2.5 (0.3 to 4.7) | 1 | 0.5 (−0.5 to 1.5) | 0.12 |
|
| |||||
|
Laboratory adverse event
| |||||
| Alanine aminotransferase | |||||
|
| |||||
| Any | 9 | 4.5 (1.6 to 7.4) | 8 | 4.0 (1.3 to 6.7) | 0.81 |
|
| |||||
| Severity grade 2 or higher | 2 | 1.0 (−0.4 to 2.4) | 1 | 0.5 (−0.5 to 1.5) | 0.62 |
|
| |||||
| Creatinine
| |||||
| Any | 2 | 1.0 (−0.4 to 2.4) | 2 | 1.0 (−0.4 to 2.4) | 1.00 |
|
| |||||
| Severity grade 2 or higher | 0 | 0.0 (0.0 to 0.0) | 0 | 0.0 (0.0 to 0.0) | — |
|
| |||||
| Hemoglobin
| |||||
| Any | 23 | 11.6 (7.1 to 16.0) | 24 | 11.9 (7.4 to 16.5) | 1.00 |
| Severity grade 2 or higher | 0 | 0.0 (0.0 to 0.0) | 5 | 2.5 (0.3 to 4.7) | 0.06 |
|
| |||||
| Lymphocytes
| |||||
| Any | 30 | 15.1 (10.1 to 20.1) | 23 | 11.4 (7.0 to 15.9) | 0.31 |
|
| |||||
| Severity grade 2 or higher | 0 | 0.0 (0.0 to 0.0) | 0 | 0.0 (0.0 to 0.0) | — |
|
| |||||
| Platelets
| |||||
| Any | 65 | 32.7 (26.2 to 39.2) | 51 | 25.4 (19.3 to 31.4) | 0.12 |
|
| |||||
| Severity grade 2 or higher | 0 | 0.0 (0.0 to 0.0) | 0 | 0.0 (0.0 to 0.0) | — |
|
| |||||
| White cells
| |||||
| Any | 36 | 18.1 (12.7 to 23.4) | 37 | 18.4 (13.0 to 23.8) | 1.00 |
|
| |||||
| Severity grade 2 or higher | 12 | 6.0 (2.7 to 9.3) | 14 | 7.0 (3.4 to 10.5) | 0.84 |
P values are for the difference between vaccine groups.
An unsolicited adverse event was an adverse event reported spontaneously by a parent or guardian.
Local reactions occurred in 99.0% of children in the vaccine group (95% CI, 96.4 to 99.0) and in 82.1% in the control group (95% CI, 76.1 to 87.1). Most local reactions consisted of swelling, typically noted only on physical examination and not of concern to parents. The most common unsolicited adverse event was subjective fever, reported in 40.2% of children in the malaria-vaccine group and 17.4% of those in the control group (P<0.001). There were no other imbalances between the two groups with respect to other, less-frequent unsolicited adverse events (Table 3). Fever was also the most common solicited symptom in both groups, recorded for 61.3% of children in the malaria-vaccine group and for 25.9% of children in the control group. Irritability or fussiness and loss of appetite were reported more frequently in the malaria-vaccine group than in the control group (Table 1 in the Supplementary Appendix).
IMMUNOGENICITY
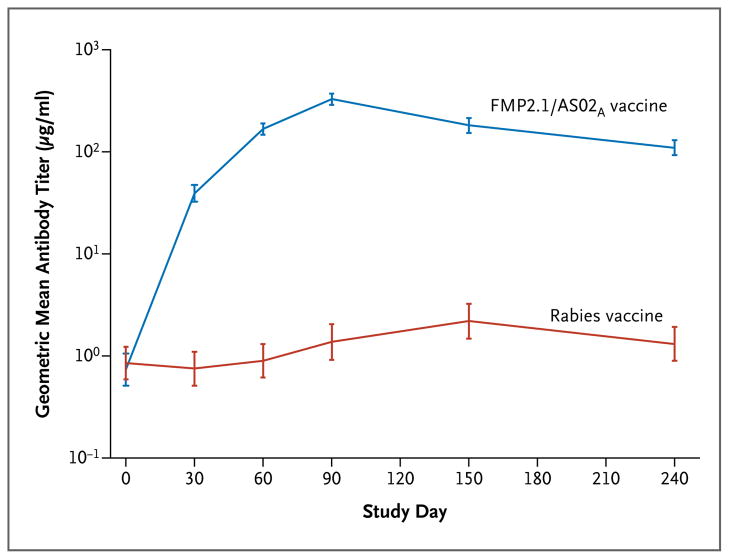
The geometric mean titer of anti-AMA1 antibodies 1 month after the third vaccination was 188,449 enzyme-linked immunosorbent assay units (EU) per milliliter (95% CI, 165,626 to 214,418) in the malaria-vaccine group and 782 EU per milliliter (95% CI, 525 to 1165) in the control group (Fig. 3). Antibody titers increased by a factor of 54 a month after the first vaccination with the malaria vaccine, peaking a month after the third vaccination, with an increase over the baseline titer by a factor of 479. The titers in the malaria-vaccine group remained significantly higher than those in the control group throughout the observation period. Anti-AMA1 antibody titers in the control group increased modestly during the malaria transmission season, rising by a factor of two by 1 month after the first vaccination and peaking at three times the baseline level by 3 months after the third vaccination.
Figure 3. Geometric Mean Titers of Antibody against Apical Membrane Antigen 1 (AMA1), According to Vaccine Group.
Immunizations were administered on study days 0, 30, and 60. I bars represent 95% confidence intervals.
Neither the AMA1 antibody titer at baseline nor the titer 1 month after the third vaccination was associated with efficacy against clinical malaria, but the increase over the baseline titer at the last measurement before the first clinical episode was associated with protection against clinical malaria, as defined according to criteria for the primary end point (hazard ratio, 0.72; 95% CI, 0.60 to 0.87; P<0.001).
DISCUSSION
The FMP2.1/AS02A malaria vaccine did not have significant efficacy against clinical malaria episodes meeting the criteria for the primary efficacy end point. However, in planned secondary analyses, the vaccine had efficacy against clinical malaria caused by parasites that were identical to the vaccine strain with respect to immunologically important polymorphisms in the vaccine antigen. Sensitivity analyses provided estimates of about 20% efficacy against both first and multiple malaria episodes diagnosed according to various definitions of clinical malaria. This finding suggests that it is possible for a malaria vaccine targeting a single blood-stage protein to provide protection against clinical malaria.
Another AMA1 vaccine was associated with a possibly increased risk of severe anemia.5 We found that recipients of the FMP2.1/AS02A vaccine did not have an increased risk of anemia but had more local reactions and other adverse events than did control-vaccine recipients. The safety and adverse-event profile of this vaccine was acceptable and similar to those of other vaccines containing the same or similar adjuvant systems.
FMP2.1/AS02A elicited sustained antibody titers. Neither baseline nor peak postimmunization antibody levels were associated with protection against clinical malaria. However, the increase in antibody levels occurring between vaccination and the first clinical episode did correlate with an increase in protection. These findings suggest that naturally acquired AMA1 antibodies might reflect recent exposure to malaria and might therefore be temporally associated with an increased risk of malaria, not as the cause but as an effect of this increased risk, providing support for a role of vaccine-induced antibodies in protection against clinical malaria. Alternatively, the postvaccination rise in antibody levels may reflect enhancement of some other protective mechanism. We previously reported that FMP2.1/AS02A elicited cellular immune responses in Malian adults.19 Studies of cellular immune responses, IgG subclass, antibody avidity, and serologic growth inhibition may identify immune correlates of protection and inform future decisions about vaccine development for clinical use.
Several factors may account for the apparent ability of FMP2.1/AS02A to provide partial protection even though other single-antigen blood-stage malaria vaccines provided none. No malaria vaccine has been designed on the basis of knowledge of the frequency of antigenic variants at the testing site, nor have previous trials been designed to detect strain-specific efficacy.6 A low frequency of parasites that were homologous to the vaccine strain most likely contributed to the lack of efficacy reported for one blood-stage vaccine.4 Although one of the most frequent AMA1 variants at our study site corresponds to the P. falciparum strain used to create FMP2.1/AS02A, improving prospects for measurable efficacy,7 our trial was underpowered to detect efficacy against strains with AMA1 completely identical to the vaccine strain, in the absence of any cross-protection. Trials of vaccines based on polymorphic antigens should be designed with consideration of strain frequencies at the study sites.
A bivalent AMA1 vaccine with aluminum hydroxide as an adjuvant, which showed no overall5 or strain-specific17 efficacy in Malian children, produced comparatively modest antibody responses (peak antibody level approximately 15 times the baseline level, as compared with an increase by a factor of 479 seen with FMP2.1/AS02A) that were short-lived, suggesting that insufficient immunogenicity contributed to the lack of efficacy.5 Use of AS02A most likely contributed to the apparent strain-specific efficacy of FMP2.1/AS02A. The development of the malaria vaccine RTS,S/AS01, which reduces the risk of clinical malaria in children,20,21 has undergone progressive improvements in adjuvant systems.22–24 Adjuvants appear to be a necessary component for efficacious subunit-protein malaria vaccines.
Previously, FMP2.1/AS02A did not prevent or delay parasitemia in volunteers with no history of malaria who were challenged by infected mosquitoes, but it did reduce parasite density before treatment.14 This result and our observation that the vaccine prevented clinical malaria caused by homologous parasites and reduced cumulative parasite density associated with all infections suggest that FMP2.1/AS02A controls parasite density by reducing the efficiency of parasitic invasion of erythrocytes.
FMP2.1/AS02A appears to have some activity against parasites with AMA1 that is not identical to the vaccine strain, since even complete efficacy against the small proportion of parasites with an AMA1 sequence that is identical to that of the vaccine strain would not account for the modest reduction in the incidence of first or multiple clinical episodes observed in secondary and exploratory analyses or for the larger reduction in episodes caused by parasites that correspond to the vaccine strain at a few immunologically important amino acid positions but that differ from the vaccine strain at other amino acid positions.7 Further analyses of molecular and immunologic correlates of protection may provide insight into the extent of cross-protection and guide the development of more effective next-generation vaccines.3
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and should not to be construed as official positions of the U.S. Department of the Army or Department of Defense.
Supported by a contract (N01AI85346) and a cooperative agreement (U19AI065683) from the National Institute of Allergy and Infectious Diseases, a grant (D43TW001589) from the Fogarty International Center, National Institutes of Health, and a contract (W81XWH-06-1-0427) from the Department of Defense and the U.S. Agency for International Development for site development and the conduct of the trial; by a contract (HH-SN272200800013C) from the National Institute of Allergy and Infectious Diseases for data management and statistical support; by grants from the U.S. Agency for International Development and the Military Infectious Diseases Research Program, Fort Detrick, MD, for vaccine production and laboratory assays; and by the Doris Duke Charitable Foundation Distinguished Clinical Scientist Award and an award from the Howard Hughes Medical Institute (to Dr. Plowe).
We thank Steven Rosenthal, Walter Jones, Abdollah Naficy, and Lee Hall of the National Institute of Allergy and Infectious Diseases for support and advice; Mariam Traore Guindo and Boubacar Kouyate for serving as local medical monitors; the study monitors, Norbert Tamm (of PPD) and Denise McKinney (of the U.S. Army Medical Materiel and Development Activity); Valerie Brown and the malaria team at EMMES; Mirjana Nesin, medical monitor, National Institute of Allergy and Infectious Diseases, and members of the data and safety monitoring board (Anna Durbin, Kathryn Edwards, Cristina Sison, Elissa Malkin, Mariam Traore Guindo, and David Diemert); Amanda Leach and the Glaxo-SmithKline Malaria Vaccine Project Team (in particular, Marie-Ange Demoitie, Yannick Vanloubbeeck, and Marc Lievens); Mahamadou Traore, Bourama Kane, Mamadou Dembele, and the Bandiagara District Hospital staff for clinical assistance; Danzele Coulibaly, Sekouba Mariko, and Moctar Traore for administrative support; Nicole Eddington, Carey Martin, and Lisa Ware for technical and administrative support; Karen Ball for regulatory support; the team of the Bandiagara Malaria Project in Bandiagara for their dedication; and the community of Bandiagara, Mali.
(Funded by the National Institute of Allergy and Infectious Diseases and others; ClinicalTrials.gov number, NCT00460525.)
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Plowe CV, Alonso P, Hoffman SL. The potential role of vaccines in the elimination of falciparum malaria and the eventual eradication of malaria. J Infect Dis. 2009;200:1646–9. doi: 10.1086/646613. [DOI] [PubMed] [Google Scholar]
- 2.A research agenda for malaria eradication: vaccines. PLoS Med. 2011;8(1):e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heppner DG, Jr, Kester KE, Ockenhouse CF, et al. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine. 2005;23:2243–50. doi: 10.1016/j.vaccine.2005.01.142. [DOI] [PubMed] [Google Scholar]
- 4.Ogutu BR, Apollo OJ, McKinney D, et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE. 2009;4(3):e4708. doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagara I, Dicko A, Ellis RD, et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine. 2009;27:3090–8. doi: 10.1016/j.vaccine.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol. 2009;31:560–73. doi: 10.1111/j.1365-3024.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takala SL, Coulibaly D, Thera MA, et al. Extreme polymorphism in a vaccine antigen and risk of clinical malaria: implications for vaccine development. Sci Transl Med. 2009;1(2):2ra5. doi: 10.1126/scitranslmed.3000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genton B, Betuela I, Felger I, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1–2b trial in Papua New Guinea. J Infect Dis. 2002;185:820–7. doi: 10.1086/339342. [DOI] [PubMed] [Google Scholar]
- 9.Enosse S, Dobaño C, Quelhas D, et al. RTS,S/AS02A malaria vaccine does not induce parasite CSP T cell epitope selection and reduces multiplicity of infection. PLoS Clin Trials. 2006;1(1):e5. doi: 10.1371/journal.pctr.0010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alloueche A, Milligan P, Conway DJ, et al. Protective efficacy of the RTS,S/AS02 Plasmodium falciparum malaria vaccine is not strain specific. Am J Trop Med Hyg. 2003;68:97–101. [PubMed] [Google Scholar]
- 11.Waitumbi JN, Anyona SB, Hunja CW, et al. Impact of RTS,S/AS02(A) and RTS,S/ AS01(B) on genotypes of P. falciparum in adults participating in a malaria vaccine clinical trial. PLoS ONE. 2009;4(11):e7849. doi: 10.1371/journal.pone.0007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta S, Lalitha PV, Ware LA, et al. Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infect Immun. 2002;70:3101–10. doi: 10.1128/IAI.70.6.3101-3110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polhemus ME, Magill AJ, Cummings JF, et al. Phase I dose escalation safety and immunogenicity trial of Plasmodium falciparum apical membrane protein (AMA-1) FMP2. 1, adjuvanted with AS02A, in malaria-naive adults at the Walter Reed Army. Institute of Research Vaccine. 2007;25:4203–12. doi: 10.1016/j.vaccine.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Spring MD, Cummings JF, Ockenhouse CF, et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS ONE. 2009;4(4):e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thera MA, Doumbo OK, Coulibaly D, et al. Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial. PLoS ONE. 2008;3(1):e1465. doi: 10.1371/journal.pone.0001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thera MA, Doumbo OK, Coulibaly D, et al. Safety and immunogenicity of an AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial. PLoS ONE. 2010;5(2):e9041. doi: 10.1371/journal.pone.0009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouattara A, Mu J, Takala-Harrison S, et al. Lack of allele-specific efficacy of a bivalent AMA1 malaria vaccine. Malar J. 2010;9:175. doi: 10.1186/1475-2875-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutta S, Lee SY, Batchelor AH, Lanar DE. Structural basis of antigenic escape of a malaria vaccine candidate. Proc Natl Acad Sci U S A. 2007;104:12488–93. doi: 10.1073/pnas.0701464104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyke KE, Daou M, Diarra I, et al. Cell-mediated immunity elicited by the blood stage malaria vaccine apical membrane antigen 1 in Malian adults: results of a Phase I randomized trial. Vaccine. 2009;27:2171–6. doi: 10.1016/j.vaccine.2009.01.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 21.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–32. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon DM, McGovern TW, Krzych U, et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis. 1995;171:1576–85. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 23.Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 24.Kester KE, McKinney DA, Tornieporth N, et al. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J Infect Dis. 2001;183:640–7. doi: 10.1086/318534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.