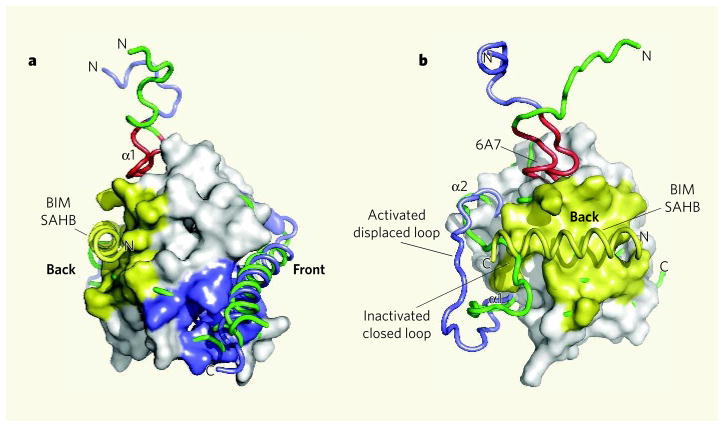
Figure 1. Activation of the BAX protein by the BIM SAHB domain.
a, The structure of BAX based on a previous study11 is shown with BIM SAHB binding at a site on the ‘back’ of the molecule (yellow). This contrasts with the expected binding of a BH3-domain helix in the conventional (at least for anti-apoptotic proteins) hydrophobic BH pocket located at the ‘front’ of the molecule (blue). b, Walensky, Tjandra and colleagues2 find that conformational changes accompany the binding of a BIM SAHB peptide to BAX. The chain containing α-helix 1 (α1) and the loop between α1 and α2 is shown before (green) and after (blue) binding of BIM SAHB. The BAX amino-terminal region 6A7, which becomes exposed on association with BIM SAHB, is shown in red. (Images prepared by T. Moldoveanu, St Jude Children’s Research Hospital.)