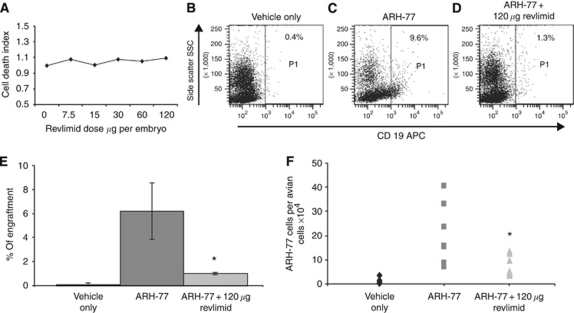
Figure 4.
Efficacy of lenalinomide (Revlimid) treatment of multiple myeloma (MM)-injected turkey embryos. (A) Different concentrations of lenalinomide were injected into turkey embryos on embryonic day 13 (E13) and bone marrow (BM) cell toxicity was assessed. No toxicity was observed up to 120 μg per embryo. ARH-77 cells were injected intravenously on E11, and embryos were treated with lenalinomide on E13. (B–D) Turkey embryos on E18. Bone marrow cells were stained with allophycocyanin (APC)-anti-human CD19. (B) A typical analysis of a vehicle-injected embryo. (C) An embryo xenografted with MM cells, and (D) a xenografted embryo treated with lenalinomide 120 μg per embryo. (E) The average engraftment when analyzed by flow cytometry or by Q-PCR (F). *P⩽0.005 compared with ARH-77-injected embryos (Student's t-test).