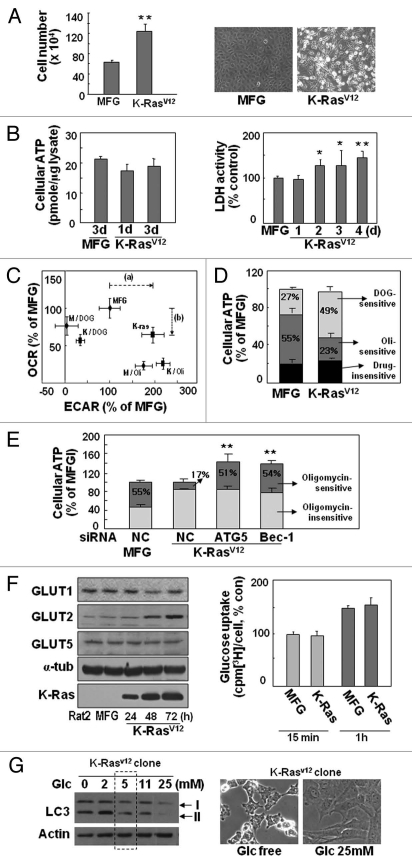
Figure 5.
K-RasV12-induced autophagy is dependent on extracellular glucose levels. (A, B and F) Rat2 cells were infected with retrovirus harboring K-RasV12 for 3 d unless indicated. (A) Cell growth rates were measured by counting trypan blue-negative cells (left part) and phase contrast cell images are shown (right part). (B) Total cellular ATP levels were estimated by using luciferin/luciferase ATP assay kit (left part) and LDH activity to oxidize NADH was monitored at 340 nm using spectrophotometry (right part). (C and D) After infected by K-RasV12 retrovirus for 3 d, Rat2 cells were treated with 2 µM oligomycin (Oli) or 25 mM 2-deoxyglucose (DOG) for 3 h. (C) Extracellular acidification rate and cellular oxygen consumption rate were simultaneously measured by Seahorse XF analyzer as described in ‘Materials and Methods’. Dotted arrows indicate lactate increase (a) and respiratory defect (b) by K-Ras. (D) Intracellular ATP levels. DOG-sensitive (glycolysis dependent) and Oli-sensitive (mitochondrial-respiration dependent) ATP production were indicated as percentage of total cellular ATP levels. (E) Intracellular ATP levels. Rat2 cells were transfected with si-ATG5 or si-Beclin 1 (Bec-1) 15 h prior to K-RasV12 infection and further incubated for 3 d, then replenished with 2 µM oligomycin containing medium for 3 h. (F) Expression levels of GLUT proteins were monitored by protein gel blot analysis (left part) and cellular glucose uptake activity was measured as described in ‘Materials and Methods’ (right part). (G) LC3-II formation of Rat2 clone stably expressing K-RasV12 in response to different concentrations of glucose in media for 3 d was monitored by protein gel blot analysis (left part) and phase contrast cell images are shown (right part).