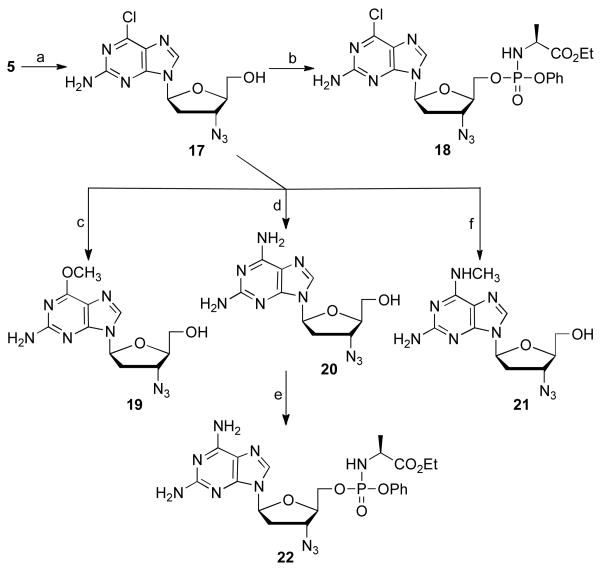
Scheme 5.
Synthesis of some l-2-amino 6-substituted 3′-azido purines. Reagents and conditions: (a) i) 2-amino-6-chloropurine, BSA, TMSOTf, acetonitrile, 85 °C, 18 h, 54%; ii) TBAF, THF, 5 h, rt, 27%; (b) t-BuMgCl, phenyl ethoxyalaninyl phosphorochloridate, THF, rt, 18 h, 40%; (c) NaOCH3, CH3OH, 80 °C, 16 h, 71%; (d) NH3/CH3OH, CH3OH, 110 °C, 18 h, 87%; (e) t-BuMgCl, phenyl ethoxyalaninyl phosphorochloridate, THF, rt, 18 h, 60%; (f) methylamine, THF, 80 °C, 18 h, 71%.