Abstract
Study Objectives:
To test the hypothesis that total sleep deprivation (TSD) slows stimulus detection and evaluation processes. Towards that end we manipulate degradation of the imperative stimulus, a manipulation well established to affect the processes of interest, in a delayed letter recognition (DLR) task and the psychomotor vigilance task (PVT), and predicted that after TSD the ordinary reaction time (RT) slowing effect of stimulus degradation would be increased. These hypotheses were only partially confirmed (see below).
Design:
Participants were exposed to 48 h of total sleep loss. The PVT and DLR were administered to the same participants. The PVT was administered 8 times —every 6 h from 12:00 on Day 1. The DLR was administered twice, at 09:00 of Day 1 and 48 h later.
Setting:
Participants were continuously monitored in a sleep laboratory.
Subjects:
26 healthy young adults enrolled. Due to dropouts and technical failures, the final n's were 20 for the DLR and 21 for the PVT.
Measurements and Results:
General linear mixed models were employed. In the DLR task there was no interaction between TSD and degradation on any variable. There was, however, a significant interaction between TSD and degradation on mean reaction time in the PVT (P = 0.01).
Conclusions:
As in our previous reports, we observe the specificity with which total sleep deprivation affects cognitive processes. One aspect of visual processing, stimulus detection, was affected by total sleep deprivation and made a significant contribution to the performance impairments observed. Another aspect of visual processing, stimulus evaluation, remained unaffected after 2 days and nights of total sleep loss.
Citation:
Rakitin BC; Tucker AM; Basner RC; Stern Y. The effects of stimulus degradation after 48 hours of total sleep deprivation. SLEEP 2012;35(1):113-121.
Keywords: Total sleep deprivation, visual processing, stimulus degradation, stimulus detection, stimulus evaluation
INTRODUCTION
Empirical evidence increasingly points to disparate vulnerabilities to total sleep deprivation among cognitive processes.1–9 For example, in the psychomotor vigilance task (PVT), a commonly employed simple reaction time task exquisitely sensitive to partial and total sleep deprivation, circadian, and other effects, the effect of 48 hours of total sleep deprivation on sustained attention is evident in slowing reaction time across the 10-min task's typical administration. In contrast, the ordinary modulation of preparation for upcoming trials across the 2- to 10-s inter-trial interval is unaffected.3,10 In another example, 48 h of total sleep deprivation does not affect memory scanning speed in a delayed letter recognition (DLR) task, although the overall reaction is substantially slowed.1,6,10 Brain imaging of the latter phenomenon indicates down-regulation of a network of brain areas concomitant with the performance decrement, including secondary visual cortex activity.6 In a follow-up study, transcranial magnetic stimulation (TMS) was applied to 2 of the regions identified by the network above, as well as to a control region. Only TMS to the lateral occipital cortex reversed this total sleep deprivation-induced impairment.11 Together these results suggest the possibility that total sleep deprivation impairs DLR performance by impairing elementary visual processing.
The goal of the present study was to examine this hypothesis by explicitly manipulating the difficulty of stimulus processing by degrading the visual stimuli. For the DLR, this degradation involved scrambling the probe-letter image (which follows a memory set of 6 letters and a retention period) and targeted the stimulus evaluation process. Because PVT performance must to some extent also rely on such processes, we applied a parallel manipulation to the PVT in order to test a similar hypothesis and generalize the results. For the PVT, the degradation involved dimming the imperative stimulus (by reducing color saturation and reducing contrast with the black background screen), and targeted stimulus detection.
In providing an information processing model of the task antecedent to the present DLR, Sternberg proposed that reaction time in the DLR task was dependent primarily on 4 processes, each of which runs to completion prior to the execution of the next.12 These 4 processes were (1) encoding of the stimulus probe, (2) serial comparison, (3) binary decision, and (4) translation and response organization. In his early work, Sternberg combined manipulations that were assumed a priori to affect one of these processing stages with a manipulation with an unknown information processing demand. If the 2 manipulations produced a statistical interaction in a factorial additive factors design, the unknown factor was assumed to have the same basis as the known factor. For example, the standard manipulation of the serial comparison stage is varying the number of items to be remembered per trial. The reaction time increases linearly with set size, data that constitute the primary evidence that success in this task is mediated by serial comparison of the probe item with the memory set. Visual degradation of the memory set stimuli, a manipulation intended to impair the stimulus encoding mechanism, also slows the RT, but does so independently of increased set-size, indicating independence of stimulus encoding and serial search processes.12 Degree of total sleep deprivation does not interact with memory set size, indicating that the locus of the total sleep deprivation effect is in one of the stages other than serial search.1,6,10
If memory scanning is not slowed by total sleep deprivation, then what process or processes does total sleep deprivation affect so as to produce the overall increase in DLR reaction time? Of the 3 remaining major candidate processes, stimulus-encoding rises to the fore owing to the results of imaging the brain during performance of the DLR task before and after total sleep deprivation. One study reported that during the probe phase there were total sleep deprivation-related decrements in extrastriate visual cortex activation as part of a larger network expressed in 17 of 18 participants.6 Other studies using other tasks have reported similar findings.13 This down-regulation of early visual processing cortex strongly suggests the possibility that total sleep deprivation negatively affects stimulus encoding and other early visual processing stages (see below) and consequently slows the portions of DLR RT unrelated to memory scanning; that is, the intercept of the RT with respect to set size. Indeed, some have hypothesized that early visual processing is a key component of total sleep deprivation related deficits in working memory capacity.13
In order to test this hypothesis we have applied Sternberg's manipulation of stimulus quality to the probe in the DLR. By applying stimulus degradation to the probe, we are not affecting stimulus encoding of memory set, but rather one of two necessary information-processing precursors to the serial comparison operation. These operations are stimulus detection and stimulus evaluation, or becoming aware of the presence of a stimulus and determining its identity (i.e., name label), respectively. The method of stimulus degradation used in the DLR here consists of adding visual noise to the probe stimulus by randomly flipping some foreground and background pixels between black and white. This process yields isoluminant stimuli that are more difficult to rapidly name. Thus we have likely affected the stimulus evaluation rather than stimulus detection process. Validation of this method requires that more degraded stimuli are processed more slowly than less degraded stimuli, a phenomenon that has been amply demonstrated previously using various methods, including the present one.14–16 Applying the logic of additive factors, the hypothesis that total sleep deprivation affects stimulus evaluation would be confirmed by finding an interaction of total sleep deprivation (i.e., pre- vs post-sleep deprivation) and stimulus degradation.
We also wished to test the hypothesis that total sleep deprivation slows the stimulus detection process. However, time and other methodological constraints prevented us from applying a second manipulation to the DLR. Rather, we applied a stimulus degradation manipulation to the PVT, given as part of the same protocol as the DLR task, and to the same participants, but at different times. The PVT is a simple vigilance RT task in which participants respond to a single stimulus (in this case a large red “X”) with a single response. In previous studies the standard administration lasted 10 min, and new stimuli were presented 2-10 sec after the previous response (the response-stimulus interval, or RSI).17 This task has a 20-plus year history of proven sensitivity to sleep pressure from partial and total sleep deprivation, as well as circadian variation.18 The simplest possible information-processing model of this task requires only stimulus detection and response selection and execution stages. However, total sleep deprivation produces a time-on-task (ToT) effect, such that increasing sleep pressure yields increasing slowing of RT across the tasks' duration (i.e., a TSD by ToT interaction).3,19 This effect suggests a loss of arousal that modulates some or all constituent processes. Similarly, a recent report demonstrated that variability in the RSI modulates the RT and that this effect is independent of up to 48 h of total sleep deprivation.3 This effect was suggested to alter preparedness for the upcoming trial, and may do so by affecting the first processes called upon in each trial, which would be stimulus detection. The above study reported a lack of interaction of ToT and RSI effects, either alone or with total sleep deprivation, which suggests that RSI and ToT affect separate stages, implying that ToT might affect response selection or execution.
The stimulus degradation manipulation applied to the PVT consisted of desaturation of the colored stimuli. This manipulation does not affect the form of the stimulus (and thereby the stimulus evaluation process) like the visual noise manipulation in the DLR, but rather simply dims the stimulus making it harder to detect. (The stimulus degradation manipulation also mandated some changes to the typical PVT dynamics. See the methods section for details.) Validation (and replication) of the hypothesis that stimulus degradation affects stimulus detection in the PVT requires that the RT to more degraded stimuli be higher than the RT to less degraded stimuli. A significant total sleep deprivation by stimulus degradation effect in the PVT would confirm the hypothesis that total sleep deprivation affects stimulus detection. Moreover, we can examine interactions between RSI, ToT, and stimulus degradation with the intent determining the information processing loci of those manipulations.
METHODS
Subjects
Participants were recruited from the Columbia University Medical Center community using flyers. All subjects were right-handed with normal or corrected-to-normal vision and screened for medical and psychiatric disorders. Subjects were additionally screened for the presence of a sleep disorder, any substance abuse, and were required to abstain from caffeine for 24 h prior to study participation and for the duration of the study. Substance abuse screening tests showed no evidence of illicit drug use in subjects. Subjects maintained a sleep log for 2 weeks prior to study participation and reported sleeping an average of 8.01 ± 0.15/h per night (minimum = 7, maximum = 9.54).
Twenty-six subjects were enrolled in the study. Three subjects discontinued participation, and one subject was dismissed from the study. Due to technical problems (one incomplete data set, one probable key inversion in the DLR, and one incomplete dataset for the PVT) the final number of subjects was 20 for the DLR (aged 24.05 ± 0.57 years; range 20-28) and 21 (aged 24.86 ± 0.68 years; range 20-31) for the PVT. Table 1 provides a description of the participants.
Table 1.
Participant demographics
| DLR | PVT | |
|---|---|---|
| N | 20 | 21 |
| Age (years) | 24.4 ± 0.64 | 24.38 ± 0.61 |
| % Female | 35% | 33.34% |
| NART IQ | 121.4 ± 0.83 | 121.5 ± 0.79 |
| Education (years) | 15.7 ± 0.27 | 15.7 ± 0.26 |
NART IQ, Nelson Adult Reading Test estimated IQ. Values for age, NART IQ and education are means ± standard errors. The PVT sample includes all 20 of the DLR participants, plus another male participant.
Informed consent, as approved by the Internal Review Board of the College of Physicians and Surgeons of Columbia University, was obtained prior to study participation and after the nature and risks of the study were explained. Subjects were paid for their participation in the study.
General Protocol
Subjects were housed in the Columbia University Sleep Center, and tested there and in the Columbia University Hatch Center for MRI Research. All participants were admitted to the protocol at 07:00 on Day 1 of the protocol and dismissed under supervision after testing on the morning of Day 3, no earlier than 11:00. Thus all participants were sleep deprived for ≥ 52 h, although the last test of interest to the current report occurred beginning at approximately hour 50 of the protocol. Subjects were supervised at all times, and polysomnographic monitoring and monitoring by sleep-lab and study personnel confirmed that they remained awake during the sleep deprivation period. When not performing cognitive tests, participants had access to the Internet, music, TV, movies, and video games, and were otherwise allowed to move about the test facilities under the supervision of study personnel.
DLR Task, Protocol, and Analyses
All subjects in all studies performed the DLR task in an fMRI scanner; here, we focus on the behavioral data. The initial test occurred at 09:00 (between the first and second administrations of the PVT) and the follow-up test occurred at the same time 48 h later (after the last administration of the PVT), to control for known circadian influences on the effects of total sleep deprivation.20 The duration of the test was approximately 35 min, and on both days followed testing of another version of the DLR (see below) of the same duration. Presentation of stimuli in the scanner employed a 3000 lumen LCD projector; rear projecting an image onto screen composed of projection-TV grade material, and viewed by the participant through a 45°-inclined mirror. Responses were obtained through a Lumitouch MRI-safe button system, interfaced with the computer via a CMU Button Box.
The DLR task used here is a common variant6,21 of item recognition tasks. The sequence of trial events was as follows: (1) Trials began with a 3-s inter-trial interval (ITI) consisting of a blank screen. (2) Presentation of the memory set consisting of 6 upper-case consonant letters in a 2 × 3 array lasted for 3 s. (3) A retention interval consisting of a blank screen lasted 7 s. (4) Presentation of a single lower case letter probe letter lasted 3 s. During the probe phase participants made a recognition judgment, responding “yes” with a right-hand key press if the probe matched any of the memory set elements and “no” with a left-hand key press otherwise. In addition to the 3-s ITI, there were also 70 2-s intervals per block that were inserted in a random fashion between trials. Thus the mean ITI was 9.2 ± 5.3 s.
The critical experimental factor was degradation of the probe, consisting of a random flipping of foreground and background pixels between black and white. Degradation either could be low (0% flipping), medium (25%), or high (50%). Degradation was varied pseudo-randomly across trials. Each of 3 experimental blocks contained 10 trials at each of the 3 degradation levels, with 5 true negative (i.e., non-matching) probes and 5 true positive (i.e., matching) probes per degradation level, yielding a total of 30 trials per degradation level per subject and 90 experimental trials in total.
Prior to the degraded-probe DLR, all participants had extensive exposure to 300 trials of a DLR task that varied memory set size (i.e., 1, 3, or 6 letters presented in the stimulus phase of each trial) and used non-degraded probes, but was in all other respects identical to the degraded-probe DLR. The first 180 trials included response-accuracy feedback; the last 90 trials were administered in the same scan session and immediately prior to the degraded-probe DLR. This variable memory-set DLR data are not analyzed here, but both the behavioral and brain-imaging data have been the subject of previous reports.8,10
Four dependent variables were computed for each combination of stimulus degradation level and session (i.e., initial and follow-up tests). Response accuracy was summarized using signal-detection theory measures of discriminability and bias. The discriminability measure was dL, given by the formula dL = ln ([H (1 – FA)]/[(1 – H) FA]), where H = hits (correct true-positive probe trials), and FA = false alarms (incorrect true-positive probe trials), and ln is the natural logarithm function. Response bias was calculated as CL, given by the formula CL = 0.5[ln ([(1 – FA)(1 – H)]/[(H)(FA)])].22,23 Speed of processing was summarized as mean RT by condition for all responses, both correct and incorrect responses are used so that the contributing trial set is the same as that used to compute dL and cL. Percent failures-to-respond (%FR) was a variable defined as the percentage of total trials for which no response was recorded (i.e., trials for which the subject exhibited a failure to respond). Four separate general linear mixed-effects models (GLMMs), with variance components and a random effect on the intercept for each subject, were used to analyze these variables. The independent variables were Day, a within-participants effect with 2 levels (baseline and follow-up), and Degradation, a within-participants effect with 3 levels (“L,” low, “M,” medium, and “H,” high degradation). Power was computed using G*Power 3.
PVT Task, Protocol, and Analyses
A computerized PVT (modeled on Dinges and Powell17) was administered upon admission to the protocol, at 12:00 Day 1, then every 6 h thereafter until 06:00 on Day 3, for a total of 9 testing sessions. The duration of the testing sessions is described below. The test was administered with a single Macintosh iBook G3 computer. Subjects responded with a space-bar press to the appearance of a red “X,” presented on the 13-inch LCD screen, which was followed by RT feedback. The “X” was degraded to relative color saturation levels of 0.25, 0.35, 0.5, 0.71, and 1 (this last value reflects the normal, non-degraded stimulus).
For this investigation we changed the PVT in several ways in order to investigate stimulus detection. For one thing, we fixed the number of trials in order to ensure that all participants would have adequate and equivalent numbers of trials for each stimulus degradation condition. The RSI varied randomly from 2-6 s. This distribution of RSI values differs from the standard 2- to 10-s RSI used in previous experiments.17,24 The reasons for the change were practical: the degradation manipulation required more trials than is typically administered post-sleep deprivation,24 so the RSI values were shortened to maintain overall task duration of approximately 7.5-9 min (see below), which is less than the typical administration length of 10 min. Our previous report suggested that this range of values was sufficient to produce a baseline RSI effect on mean RT. The initial PVT session was administered before 09:30 of the first day after a normal night of sleep at home. Eight additional sessions were administered every 6 h during the sleep deprivation protocol beginning at noon of the first day and extending until 06:00 of the third and final day, after 48 h of total sleep deprivation. Data from the last 8 sessions are analyzed here. One hundred trials were presented per session, 20 for each value of stimulus degradation. Each value of degradation was presented 4 times in each consecutive set of 20 trials. The mean task duration was 7.58 ± 0.21 min on Day 1 (see below), and 8.69 ± 0.59 min on Day 2, both somewhat shorter than the typical 10-min PVT duration, but long enough to be sensitive to total sleep deprivation-induced response slowing.25
Three variables were used to summarize PVT task performance. The mean RT, excluding responses classified as errors of omission or commission, summarized the central tendency of response speed. Percent commission errors (%CE) were defined as the percentage of the total number of responses with RT < 100 ms, and percent lapses (%Lapses) were defined as the percentage of the total number of responses with RT > 500 ms (i.e., errors of omission). Six separate GLMMs, with variance components and a random effect on the intercept for each subject, were used to analyze these variables. All models included Day, a within-participants factor with 2 levels (Day 1, sessions 2-5, and Day 2, sessions 6-9), and Degradation, a within-participants factor with 5 levels (0.25, 0.35, 0.5, 0.71, and 1). Three of the models included ToT, a within-participants factor with 2 levels (minutes 1-5, and minute 5–task termination). The other 3 models included RSI, a within-participants factor with 2 levels (“S,” or short, RSI = 2-3; “M,” or medium, RSI = 4-6). RSI and ToT could not be included in the same models because too many cells did not contain data when crossed with Degradation. All models included planned log-linear contrasts over Degradation (i.e. weights −2, −1, 0, 1, 2 over the log-spaced degradation values).
RESULTS
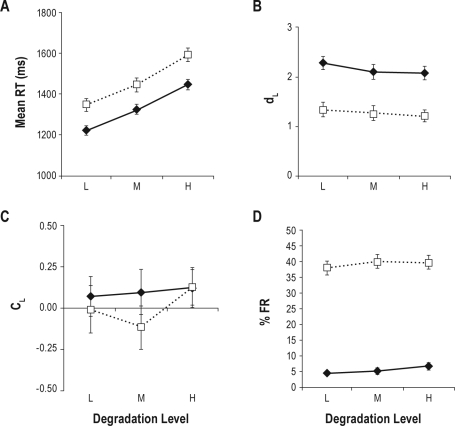
The results for the DLR task are illustrated in Figure 1, and the complete results of the GLMMs are presented in Table 2. There were significant effects of total sleep deprivation on all 4 variables (see main effect of “Day” in Table 2), such that RT was slowed (panel A), discriminability diminished (panel B), thresholds were somewhat reduced (panel C), and the percentage of failures-to-respond was starkly increased (panel D). These effects are similar to those reported previously for the effects of total sleep deprivation on a variable set-size DLR.6 There was a strong main effect of stimulus degradation (see main effect of “Deg” in Table 2) such that RT increased in a more or less linear manner with increased degradation. This test validates the degradation manipulation. However, there was no interaction between the effects of total sleep deprivation and degradation for RT or any other variable, suggesting that the mechanism by which total sleep deprivation increases the DLR mean RT is not via slowing of stimulus evaluation. An analysis of the power of a one-degree of freedom contrast over degradation within this interaction, computed as a one-tailed t(95), indicated that this design was capable of detecting an effect of dz = 0.257, defined as a small effect by Cohen's criteria. The implications of this analysis will be elaborated in the discussion section.
Figure 1.
Delayed letter recognition (DLR) task data for each of the 4 dependent variables. Solid lines are data from Day 1 (pre-sleep deprivation) and dotted lines are data from Day 2 (post sleep deprivation). “L,” “M,” and “H,” indicate low, medium, and high levels of the stimulus degradation manipulation, respectively. The effect of Day is significant for all variables. The effect of Degradation is significant only for mean reaction time (RT) (Panel A), although there are no significant interactions of Day and Degradation for RT or any other variable.
Table 2.
Mixed-effects model results for DLR task variables
| df | Variable |
||||
|---|---|---|---|---|---|
| RT | dL | CL | %FR | ||
| Tests of Fixed Effects | |||||
| Day | 1, 95 | 33.82 | 100.93 | 6.26 | 277.98 |
| Deg | 2, 95 | 27.56 | 1.25 | 1.23 | 0.46 |
| Day * Deg | 2, 95 | 0.09 | 0.14 | 0.26 | 0.03 |
| Orthogonal Contrasts | |||||
| Day * Deg(H – [M + L]) | 95 | 0.32 | 0.10 | 0.71 | −0.13 |
| Day * Deg(M - L) | 95 | −0.26 | −0.52 | 0.09 | −0.23 |
Table values are F for tests of fixed effects, and t for planned contrasts. Values in italics are significant at the α = 0.05 level. Deg, degradation effect; df, degrees of freedom; RT, mean reaction time; %FR, percent failure-to-respond. For planned contrast notation “H,” “M,” and “L,” indicate high, medium, and low degradation levels, respectively.
The results from the 2 GLMM analyses of the PVT task data are presented in Table 3. In both models significant effects of Day resulted in increased RT, %CE, and %Lapses. ToT had a significant effect on RT, as RT slowed on average from the first 5 min to the remainder of the task. This effect was larger on Day 2 than on Day 1 (i.e., the Day by ToT interaction was significant), replicating the well-documented deleterious effect of total sleep deprivation on vigilance.19,24 ToT also increased the %Lapses overall and in interaction with total sleep deprivation. RSI significantly affected all 3 dependent measures: RT was slowest, %CE was least, and %Lapses was greatest at the shortest RSI. There were no significant interactions between RSI and Day for any of the variables, replicating our previously published result24 that trial-by-trial preparation is unaffected by 48 h of total sleep deprivation, in contrast with vigilant attention (ToT).
Table 3.
Mixed-effects model results for PVT task variables
| df | C = ToT |
C = RSI |
|||||
|---|---|---|---|---|---|---|---|
| RT | %CE | %Lapses | RT | %CE | %Lapses | ||
| Tests of Fixed Effects | |||||||
| Day | 1, 380 | 83.84 | 10.24 | 91.05 | 78.95 | 4.70 | 79.14 |
| C | 1, 380 | 14.95 | 0.75 | 30.1 | 539.67 | 44.37 | 87.81 |
| Day * C | 1, 380 | 7.51 | 5.70 | 23.06 | 2.43 | 1.22 | 0.53 |
| Deg | 4, 380 | 5.90 | 0.37 | 2.50 | 6.91 | 0.51 | 4.47 |
| Day * Deg | 4, 380 | 1.50 | 0.00 | 1.51 | 1.66 | 0.09 | 1.19 |
| C * Deg | 4, 380 | 0.80 | 0.17 | 0.29 | 1.64 | 0.38 | 1.26 |
| Day * C * Deg | 4, 380 | 0.73 | 0.05 | 0.93 | 1.13 | 0.26 | 0.30 |
| Log Linear Contrasts | |||||||
| Deg | 1, 380 | 21.55 | 0.17 | 8.80 | 24.99 | 0.15 | 15.67 |
| Deg * Day | 1, 380 | 4.19 | 0.01 | 1.01 | 4.34 | 0.01 | 0.73 |
| Deg * C | 1, 380 | 1.32 | 0.00 | 0.39 | 5.76 | 0.17 | 4.49 |
| Deg * Day * C | 1, 380 | 1.73 | 0.05 | 0.00 | 0.95 | 0.18 | 0.76 |
Table values are F for tests of fixed effects and for planned contrasts. Values in italics are significant at the α = 0.05 level. Deg, degradation level; C, either ToT effect or RSI effect as noted in column headers; ToT, Time on task; RSI, response-stimulus interval; df, degrees of freedom; RT, mean reaction time; %CE, percent commission errors; %Lapses, percent lapses.
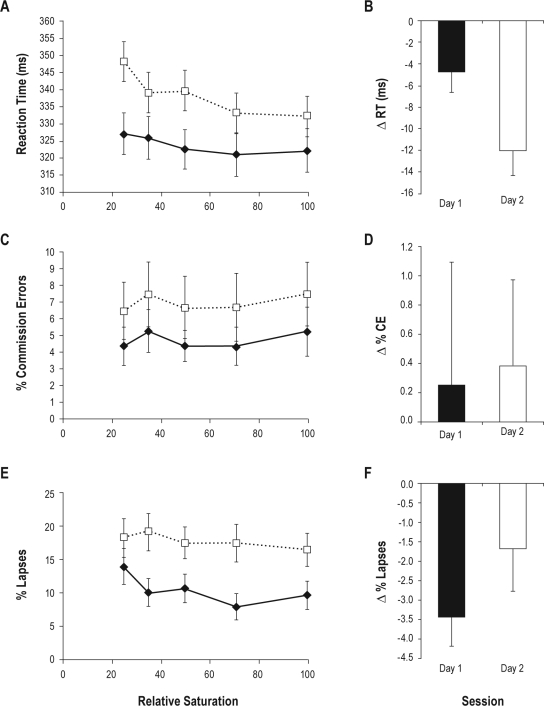
The effects of degradation as specified by the log-linear contrasts were consistent between the 2 models, and Figure 2 presents the Degradation effects as a function of Day in the ToT model. Figure 2 panels A, C, and E show the point Estimates for every level of degradation within Day, while panels B, D, and F show the least-squares estimates (i.e., model-predicted values) of the change in the variable across levels of Degradation for each Day. The results from the RSI model were qualitatively similar, and are omitted for brevity. The main effect of Degradation (in both models) was to increase RT and %Lapses for the most degraded stimuli. These results validate the current degradation manipulation. The effect of the interaction of Degradation and Day on RT (only, and in both models), reflecting greater RT slowing for more degraded stimuli on Day 2 compared to Day 1, is a critical positive result, indicating that total sleep deprivation affects the stimulus detection process. An analysis of the power of a one-degree of freedom contrast over degradation within this interaction, computed as a one-tailed t(380), indicated that this design was capable of detecting an effect of dz = 0.144, defined as a small effect by Cohen's criteria, and the obtained effect size assuming a correlation of 0.5 for the degradation effect between days was dz = 0.1763. There were no higher order interactions (either Deg * Day * ToT or Deg * Day * RSI) that would indicate that total sleep deprivation-related modulation of stimulus detection is moderated by depletion of an energetic resource (i.e., ToT26,27). There was however an interaction between Degradation and RSI, such that longer RSI values were associated with 9.7 ms of additional degradation-related slowing.
Figure 2.
Psychomotor vigilance task (PVT) task results for each of the 3 dependent variables. Panels A, C, and E plot the point-estimate cell means and standard errors for mean reaction time (RT), percent commission errors (%CE), and percent lapses (%Lapses), respectively, by degradation level for Day 1 (solid line) and Day 2 (dotted line). The main effect of Degradation is significant for RT (Panel A) and %Lapses (Panel E), and the main effect of Day is significant for all 3 variables. Panels B, D, and F present the least-square means and standard errors of the log-linear contrast (i.e., the change in the variable from high to low degradation, denoted by the symbol “▵”) from the ToT model, for mean RT, %CE, and %Lapses, respectively. This contrast is equivalent to the linear change in RT as a function of degradation ignoring the unequal spacing of independent variable values. The Day by Degradation (Day * Deg) interaction is significant only for RT (Panel B). Negative values indicate a reduction in the variable from high to low degradation.
DISCUSSION
In the present report, two different manipulations of stimulus quality were applied to two different tasks given to the same participants in a study of 48 hours of total sleep deprivation. The goal was to determine whether total sleep deprivation affects cognitive task performance by altering either or both early visual processes, stimulus detection and stimulus evaluation. The analytic method used to make this inference was the additive factors method. That is, we hoped to detect total sleep deprivation-related disturbances of early visual processing in the form of a statistical interaction between total sleep deprivation and the manipulation of stimulus degradation, such that stimulus degradation effects would be larger after total sleep deprivation compared to baseline. The results of these experiments can be summarized as follows: While both manipulations were valid to the extent that greater stimulus degradation led to greater impairment of task performance, only in the PVT task was the interaction between total sleep deprivation and stimulus degradation significant. Thus we conclude based on the present data that after 48 hours of total sleep deprivation, impairment to the stimulus detection process, but not the stimulus evaluation process, is a significant contributing factor to total sleep deprivation-related cognitive task performance impairment.
An important potential limitation to the apparent dissociation between the effects of total sleep deprivation on stimulus detection and evaluation is that the two different tasks used to assess the two effects differed in their sensitivity. That is, while the DLR task design was sufficient to detect effects defined as small by Cohen,28 the sensitivity is not sufficient to detect an effect the size of the Day × Degradation interaction discovered in the PVT. However, the main effect of Degradation in the DLR task (mean = 150.12, SE = 22.98, dz = 0.6667) is enormous compared to the main effect of degradation in the PVT task (mean = 8.34, SE = 1.8, dz = 0.2439), a fact that should be considered when gauging the potential extent to which total sleep deprivation could modulate the stimulus degradation effect. Accordingly, we can compute the expected effect size of the DLR Day × Degradation as a proportion of the DLR Degradation effect size assuming that that proportion is equal to the (Day × Degradation)/Degradation effect size ratio in the PVT task. This works out to an effect size of dz = 0.4819, which easily exceeds the sensitivity of the DLR task as described in the results section. This computation indicates that the DLR task had sufficient power to detect a total sleep deprivation-related change in the stimulus degradation effect equal to that observed in the PVT task after accounting for baseline differences in the magnitude of the main stimulus degradation effect. Nonetheless, the implied dissociation between the effects of total sleep deprivation on the two early visual processes would be best supported by a replication study that included both stimulus degradation manipulations in a single, factorial experimental design with sensitivity equal to that of the current PVT design.
There have been a small number of studies that have employed stimulus degradation to elaborate the effects of total sleep deprivation and which bear comparison to the present data. Work by Sanders et al. employed stimulus degradation and intensity manipulations similar to the degradation manipulations employed here in the DLR and PVT, respectively.29 Using the additive factors logic that report concluded that the feature extraction (synonymous with stimulus evaluation) stage of a three-choice RT task was selectively impaired by one night's sleep loss, based on a total sleep deprivation × degradation interaction on the mean RT. Moreover, there was additional modulation of this interaction by time on task over the 20-minute test session, such that the degradation effect after total sleep deprivation was larger at the end of the session. This pattern suggested to Sanders an “energetic” effect as opposed to a “direct” effect of total sleep deprivation on feature extraction.27 That is, Sanders theorized that the effect of total sleep deprivation was to diminish a limited resource needed by feature extraction (and not other processes), as opposed to directly impairing feature extraction. A later study by Smulders et al. used a task and manipulation very similar to the previous study, but failed to find an effect of 28 hours of sleep deprivation in interaction with stimulus degradation on the mean reaction time.26 That report suggested that the failure to replicate Sander's et al. might have been due to its use of blocked presentation of degraded and non-degraded stimuli.26
In comparison, the current study also failed to find a total sleep deprivation-related stimulus degradation effect (i.e., an interaction of study-day and degradation level) in the DLR task, even though different levels of degradation were mixed within blocks, a condition that, according to Smulders, should maximize the likelihood of finding such an effect. However, we did find the expected large main effect of degradation on the mean RT, and so consider the present DLR experiment a fair test to replicate and extend those previous studies to this more complicated task and a longer duration of total sleep deprivation. As such, our results comport more with those of Smulders et al.26 rather than Sanders et al.,29 in suggesting that the effect of total sleep deprivation on stimulus evaluation is minimal. However, this conclusion is necessarily mitigated by the many task, manipulation, procedure, and protocol differences among the studies. Similarly, our current PVT results indicating that total sleep deprivation impairs the earlier stimulus detection processes contrasts with those of Sanders et al., for reasons that are not at all clear.29
More generally we may ask how and why stimulus evaluation is less impaired by fatigue than the stimulus detection, when the former is judged a more complex process by its longer duration? Could it be that sleep deprived individuals can compensate for deficits in the more complex process of stimulus evaluation but cannot compensate for deficits in the simpler process of stimulus detection? In the context of non-sleep deprived individuals, stimulus evaluation is thought to be susceptible to top-down control while stimulus detection does not appear to be.30,31 Drummond et al.32 posited that top-down compensatory processes help preserve performance during sleep deprivation, but that the engagement of these processes was contingent upon the “cognitive demands inherent in a task.”32 Since then, other investigations have provided additional support for the idea that compensation occurs during sleep deprivation for more complex, but not for simpler tasks. For example, Chee and Choo found that during sleep deprivation prefrontal and thalamic activation increased more for a working memory task that required manipulation of items than for one that merely required maintenance.33 In a similar vein, Drummond et al. reported that as task demands increased on a logical reasoning task during sleep deprivation, compensatory responses of prefrontal and inferior parietal regions were increased.34 Finally, Chuah et al. found that for a go/no-go task, those individuals more resistant to sleep deprivation were able to activate right ventrolateral prefrontal and insula regions more during the more difficult no-go trials requiring active response inhibition.35 Our results are in agreement with this general pattern applied, however, to more or less simple component cognitive processes within tasks, instead of between more or less simple cognitive tasks.
Another potential explanation comes from local sleep theory, which posits that neuronal assemblies can sleep even while the rest of the brain remains awake.36 Applying this theory to the cognitive deficits seen in total sleep deprivation we can predict that those processes used more continuously, such as actively maintaining working memory items over a long retention interval, will be more impaired than will more transient processes, such as those involved in scanning working memory immediately after a given probe item appears.1 In this light and turning to the current results, processes involved in stimulus detection have to be engaged in the PVT task for up to 10 seconds until the stimulus appears, while stimulus evaluation processes only briefly come online after the stimulus has been successfully detected. Thus, stimulus evaluation processes may have more chances for rest between intermittent task demands, and this is why they may be less impaired by total sleep deprivation.
A recent paper by Chee et al. integrates a number of concepts that may shed light on this.15 That neuroimaging study crossed manipulations of visual attention with perceptual difficulty, the latter being a variation in contrast related to the manipulation used here in the PVT. Behaviorally, Chee's result does not replicate the present result because while there was a strong effect of contrast on RT, there was no contrast by TSD interaction, although there was a trend for TSD participants to respond more slowly in low contrast conditions. This failure to replicate may be due to inadequate sleep deprivation (< 24 h), power issues related to sample size, or perhaps their practice of removing long RTs from the distribution. More interesting was the finding that contrast varies V1 activation, corroborating previous reports,37 but does not vary extrastriate activation. A separate manipulation of top-down attention had the opposite effect and interacted with TSD. Thus it seems that top-down attention effects may help TSD participants compensate for some TSD-related visual processing changes, but not those dependent on V1 like stimulus detection. This in turn would make behavioral discovery of sleep deprivation-related changes to extrastriate-dependent processes like stimulus evaluation more subject to inter-study factors (like task difficulty) affecting top-down visual attention.
To summarize, the present study produced behavioral evidence for total sleep deprivation associated impairment to stimulus detection, but not stimulus evaluation, in a larger sample with greater statistical power and longer total sleep deprivation than has been employed previously. This finding has safety and human factors implications, as it suggests that while having a properly lit console for work always helps boost performance, it may become even more important to ensure that work stations employ adequately bright signals if workers are likely to be operating under conditions of total sleep deprivation and/or at off-peak circadian times. Further, transmitting safety-relevant signals in multiple modalities—as auditory noises as well as visual signals—may help sleep deprived individuals compensate for visual deficits. Nonetheless, variation between reports leaves open the issues of the nature and extent of the impact on total sleep deprivation on early visual processing.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Stern has received research support from Bayer, Elan, Eli Lilly, and Janssen AI and has participated in a speaking engagement for Servier. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the Defense Advanced Research Projects Agency (DARPA) grant DAAD 19-02-01-01147 and National Institute of Aging (NIA) grant T32 AG00261. The authors thank Oksana Tatarina and Diane Abela for their assistance with data collection.
ABBREVIATIONS
- %CE
percent commission errors
- %FR
percent failures-to-respond
- %Lapses
percent lapses
- DLR
delayed letter recognition
- TSD
total sleep deprivation
- GLMM
general linear mixed-effects model
- PVT
psychomotor vigilance task
- RSI
response-stimulus interval
- RT
reaction time
- ToT
time on task
REFERENCES
- 1.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HPA. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33:47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner TH, Drummond SPA, Salamat JS, Brown GG. Effects of 42 hr of total sleep deprivation on component processes of verbal working memory. Neuropsychology. 2007;21:787–95. doi: 10.1037/0894-4105.21.6.787. [DOI] [PubMed] [Google Scholar]
- 3.Tucker AM, Basner RC, Stern Y, Rakitin BC. The variable response-stimulus interval effect and sleep deprivation: An unexplored aspect of psychomotor vigilance task performance. Sleep. 2009;32:1393–5. doi: 10.1093/sleep/32.10.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennings JR, Monk TH, van der Molen MW. Sleep deprivation influences some but not all processes of supervisory attention. Psychol Sci. 2003;14:473–9. doi: 10.1111/1467-9280.02456. [DOI] [PubMed] [Google Scholar]
- 5.Heuer H, Kohlisch O, Klein W. The effects of sleep deprivation on the generation of random sequences of key-presses, numbers and nouns. Q J Exp Psychol (Colchester) 2005;58A:275–307. doi: 10.1080/02724980343000855. [DOI] [PubMed] [Google Scholar]
- 6.Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2004;18:306–21. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey DG, Kramer AF, Stanny RR. Influence of extended wakefulness on automatic and nonautomatic processing. Hum Factors. 1994;36:652–69. doi: 10.1177/001872089403600407. [DOI] [PubMed] [Google Scholar]
- 8.Tucker AM, Rakitin BC, Basner RC, Gazes Y, Steffener J, Stern Y. fMRI activation during failures to respond key to understanding performance changes with sleep deprivation. Behav Brain Res. 2011;218:73–9. doi: 10.1016/j.bbr.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Searching night and day: a dissociation of effects of circadian phase and time awake on visual selective attention and vigilance. Psychol Sci. 2003;14:549–57. doi: 10.1046/j.0956-7976.2003.psci_1464.x. [DOI] [PubMed] [Google Scholar]
- 10.Tucker AM, Stern Y, Basner RC, Rakitin BC. The prefrontal model revisited: double dissociations between young sleep deprived and elderly subjects on cognitive components of performance. Sleep. 2011;34:1039–50. doi: 10.5665/SLEEP.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luber B, Stanford AD, Bulow P, et al. Remediation of sleep-deprivation induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb Cortex. 2008;18:2077–85. doi: 10.1093/cercor/bhm231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sternberg S. Memory-scanning: Mental processes revealed by reaction-time experiments. Am Sci. 1969;57:421–57. [PubMed] [Google Scholar]
- 13.Chee MWL, Chuah YML. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. PNAS. 2007;104:9487–92. doi: 10.1073/pnas.0610712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternberg S. The discovery of processing stages: extensions of Donders' method. Acta Psychol (Amst) 1969;30:276–315. [Google Scholar]
- 15.Chee MW, Tan JC. Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals. Neuroimage. 2010;51:835–43. doi: 10.1016/j.neuroimage.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–80. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 17.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Ins C. 1985;17:652–5. [Google Scholar]
- 18.Dorrian J, Rogers N, Dinges DF. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep deprivation: clinical issues, pharmacology and sleep loss effects. New York: Marcel Dekker, Inc.; 2005. [Google Scholar]
- 19.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: Evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 20.Van Dongen HPA, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clin Sports Med. 2005;24:237–49. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Awh E, Smith EE, Jonides J. Human rehearsal processes and the frontal lobes: PET evidence. Ann N Y Acad Sci. 1995;769:97–117. doi: 10.1111/j.1749-6632.1995.tb38134.x. [DOI] [PubMed] [Google Scholar]
- 22.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Rakitin BC, Nambisan R, Habeck C, Stern Y. The response-signal method reveals age-related changes in object working memory. Psychol Aging. 2008;23:315–29. doi: 10.1037/0882-7974.23.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucker AM, Basner RC, Stern Y, Rakitin BC. The variable response-stimulus interval effect and sleep deprivation: an unexplored aspect of psychomotor vigilance task performance. Sleep. 2009;32:1393–5. doi: 10.1093/sleep/32.10.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loh S, Lamond N, Dorrian J, Roach G, Dawson D. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behav Res Methods Instrum Comput. 2004;36:339–46. doi: 10.3758/bf03195580. [DOI] [PubMed] [Google Scholar]
- 26.Smulders FTY, Kenemans JL, Jonkman LM, Kok A. The effects of sleep loss on task performance and the electroencephalogram in young and elderly subjects. Biol Psychol. 1997;45:217–39. doi: 10.1016/s0301-0511(96)05229-5. [DOI] [PubMed] [Google Scholar]
- 27.Sanders AF. Towards a model of stress and human performance. Acta Psychol (Amst) 1983;53:61–97. doi: 10.1016/0001-6918(83)90016-1. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical power analysis for the behavioral sciences. 2nd Ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 29.Sanders AF, Wijnen JLC, van Arkel AE. An additive factor analysis of the effects of sleep loss on reaction processes. Acta Psychol (Amst) 1982;51:41–59. doi: 10.1016/0001-6918(82)90018-x. [DOI] [PubMed] [Google Scholar]
- 30.Martinez A, Anllo-Vento L, Sereno MI, et al. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci. 1999;2:364. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- 31.Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23:3990–8. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drummond SPA, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:S68–S73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- 33.Chee MWL, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drummond SPA, Brown GG, Salamat JS, Gillin JC. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004;27:445–51. [PubMed] [Google Scholar]
- 35.Chuah YM, Venkatraman V, Dinges DF, Chee MWL. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26:7156–62. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krueger JM, Rector DM, Roy S, Van Dongen HPA, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–9. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tootell RB, Reppas JB, Kwong KK, et al. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci. 1995;15:3215–30. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]