Abstract
The basolateral nucleus of the amygdala (BLA) is a key brain region regulating behavioral changes following stressful events, including social defeat. Previous research has shown that activation of serotonin (5-HT) 1A receptors in the BLA reduces conditioned fear and anxiety-like behavior. The objective of this study was to test whether 5-HT1A receptors in the BLA contribute to conditioned defeat in male Syrian hamsters (Mesocricetus auratus). We tested whether injection of the selective 5-HT1A receptor agonist flesinoxan (400 ng, 800 ng, or 1200 ng in 200 nl saline) into the BLA prior to social defeat would reduce the acquisition of conditioned defeat, and whether a similar injection prior to testing would reduce the expression of conditioned defeat. We also tested whether injection of the selective 5-HT1A receptor antagonist WAY-100635 (400 ng or 1600 ng in 200 nl saline) into the BLA prior to social defeat would enhance the acquisition of conditioned defeat, and whether a similar injection prior to testing would enhance the expression of conditioned defeat. We found that injection of flesinoxan into the BLA decreased both the acquisition and expression of conditioned defeat. However, injection of WAY-100635 into the BLA did not alter the acquisition or expression of conditioned defeat. These data indicate that pharmacological activation of 5-HT1A receptors in the BLA is sufficient to impair the acquisition and expression of conditioned defeat. Our results suggest that pharmacological treatments that activate 5-HT1A receptors in the BLA are capable of reducing the development of stress-induced changes in behavior.
Keywords: social defeat, serotonin, stress, anxiety, fear, defensive behavior
1. Introduction
In Westernized societies, psychosocial stressors are more commonly experienced than physical stressors and are a contributing factor in the onset of psychiatric disorders such as depression (Heim and Nemeroff, 2001; Kendler et al., 1999) and post-traumatic stress disorder (Kuo et al., 2003; Risbrough and Stein, 2006; Vermetten and Bremner, 2002). In animal models, psychosocial stressors such as social defeat produce robust activation of the HPA axis (Blanchard et al., 1995; Huhman et al., 1992; Koolhaas et al., 1997). Social defeat also leads to marked behavioral changes including increased depression- and anxiety-like behavior (Berton et al., 1998; Frischknecht et al., 1982; Heinrichs et al., 1992; Keeney et al., 2006; Krishnan et al., 2007). In this study, we use a social defeat model in Syrian hamsters called conditioned defeat, in which a single social defeat results in a loss of normal territorial aggression and an increase in submissive and defensive behavior in later non-aggressive social encounters. Acute social defeat paradigms such as conditioned defeat are valuable partly because they provide an ethologically relevant model for investigating the neural mechanisms underlying stress-induced changes in behavior.
The basolateral complex of the amygdala (BLA) is a critical neural structure underlying both conditioned defeat and conditioned fear. Pharmacological blockade of NMDA receptors in the BLA blocks the acquisition of fear-potentiated startle (Campeau et al., 1992; Gerwitz and Davis, 1997), conditioned freezing (Fanselow and Kim, 1994), and conditioned defeat (Jasnow et al., 2004). Also, the NR2B subunit of the NMDA receptor in the BLA plays a critical role in the neural signaling that underlies the acquisition of conditioned fear and conditioned defeat (Day et al., 2011; Rodrigues et al., 2001; Tang et al., 1999). Over-expression of CREB in the BLA using viral vector-mediated gene transfer enhances the acquisition of both fear-potentiated startle (Josselyn et al., 2001) and conditioned defeat (Jasnow et al., 2005). Finally, blocking protein synthesis in the BLA with anisomycin impairs the acquisition of conditioned freezing (Schafe and LeDoux, 2000) and conditioned defeat (Markham et al., 2010; Markham and Huhman, 2008). In sum, these data suggest that the neurochemical signals in the BLA that regulate the formation of conditioned defeat are similar to those that regulate the formation of conditioned fear.
One important difference between conditioned fear and conditioned defeat appears to be the role of serotonin (5-HT). The 5-HT system plays a key role in the etiology and treatment of stress-related mental illness (Harvey et al., 2004; Vieweg et al., 2006). The 5-HT1A receptor is also an important factor in the psychopathology underlying stress-related mental illness, and some novel pharmacological treatments for affective disorders target the 5-HT1A receptor (Dawson and Watson, 2009; Savitz et al., 2009). The 5-HT1A receptor can be expressed as a somatodendritic autoreceptor in the dorsal raphe nucleus (DRN) or as a postsynaptic heteroreceptor in the forebrain. In both cases, the 5-HT1A receptor produces hyperpolarization (Barnes and Sharp, 1999; Hoyer et al., 2002). Although early studies indicated that 5-HT1A receptors did not play an important role in fear-potentiated startle (Davis et al., 1988; Melia and Davis, 1991), later research found that administration of a 5-HT1A receptor partial agonist reduced the expression of fear-potentiated startle (Risbrough et al., 2003). More recently studies have suggested that 5-HT signaling in the hippocampus and amygdala modulates conditioned fear (Almada et al., 2009; Li et al., 2006). Activation of 5-HT1A postsynaptic receptors in the dorsal hippocampus (Li et al., 2006; Stiedl et al., 2000), dorsal periaqueductal gray (Broiz et al., 2008), and amygdala (Li et al., 2006) reduces the expression of fear-conditioned behaviors. Although these studies indicate that 5-HT1A receptors modulate the expression of conditioned fear, little research is available on whether 5-HT1A receptors modulate the formation of conditioned fear. It is noteworthy that injection of a 5-HT1A receptor agonist into the dorsal hippocampus prior to training was shown to impair fear conditioning, indicating that activation of 5-HT1A receptors in the hippocampus is sufficient to disrupt the formation of fear memories (Stiedl et al., 2000). Recently, we have shown that pharmacological activation of 5-HT1A autoreceptors in the DRN disrupts both the acquisition and expression of conditioned defeat (Cooper et al., 2008). This supports the idea that 5-HT signaling in the forebrain can modulate conditioned defeat. Thus, the conditioned defeat model provides a rare opportunity to investigate whether 5-HT1A receptor signaling in the BLA modulates the formation of memories for aversive events.
The goal of the current study was to determine whether 5-HT1A receptors are part of the neural circuitry in the BLA controlling the acquisition and expression of conditioned defeat. We hypothesized that injection of a 5-HT1A receptor agonist into the BLA would decrease both the acquisition and expression of conditioned defeat and that injection of a 5-HT1A receptor antagonist into the BLA would facilitate the acquisition and expression of conditioned defeat.
2. Material and methods
2.1 Subjects
We used male Syrian hamsters (Mesocricetus auratus) that weighed 120–140 g (3–4 months) at the start of the study. Older hamsters (160–180 g, >6 months) were individually housed and used as resident aggressors (RAs) for social defeat training. Younger hamsters (90–100g, approx 2 months) were group-housed (4 per cage) and used as non-aggressive intruders for conditioned defeat testing. All animals were purchased from Charles River Laboratories and were housed in polycarbonate cages (12 cm × 27 cm × 16 cm) with corncob bedding, cotton nesting materials, and wire mesh tops. Food and water were available ad libitum. Cages were not changed for one week prior to training to allow individuals to scent mark their territory. Animals were housed in a temperature controlled room (21 ± 2 °C) and kept on a 14:10 hr light:dark cycle. All procedures were approved by the UT Institutional Animal Care and Use Committee and follow the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Stereotaxic Surgery
Hamsters were anesthetized with isoflurane and stereotaxically implanted bilaterally with 26-gauge guide cannulae aimed at the BLA. The stereotaxic coordinates were 0.4 mm posterior and 3.9 mm lateral to bregma, and 2.2 mm below dura. During microinjection, a 33-gauge injection needle was inserted that projected 4.0 mm below the guide cannula for a final projection of 6.2 mm below dura. After surgery, dummy stylets that projected 0.1 mm below the guide cannulae were inserted into the cannulae to maintain patency. Animals were given 10–14 days to recover from surgery before behavioral experiments and were handled daily.
2.3 Conditioned Defeat Protocol
2.3.1 Social Defeat Training
Social defeat training consisted of a single aggressive encounter in the cage of a RA. In Experiments 1 and 2 we expected flesinoxan to decrease conditioned defeat behavior and subjects received a 15 min social defeat to avoid a floor effect on later submissive/defensive behavior at testing. In Experiments 3 and 4 we expected WAY-100635 to increase conditioned defeat behavior and subjects received a suboptimal 5 min social defeat to avoid a ceiling effect on later submissive/defensive behavior at testing. When drugs were given prior to social defeat, defeats were digitally recorded and the behavior of the RA was quantified later using Noldus Observer (Noldus Information Technology, Wageningen, Netherlands). We quantified the latency to first attack, total number of attacks, and total duration of aggression displayed. Any animal injured such that it bled was treated and removed from the study (1 animal total, 0.4% of subjects). To evaluate whether drugs altered behavior at testing in the absence of social defeat, we included no defeat control groups. No defeat control animals were placed in the dirty, empty cage of a RA for 5 or 15 min so that they experienced the same olfactory cues and novel environment as the defeated animals.
2.3.2 Behavioral Testing
Behavioral testing occurred 24 h after social defeat training and consisted of a 5 min social interaction test, during which a non-aggressive intruder was placed in the subject’s cage. Non-aggressive intruders are younger, group-housed animals that display social and nonsocial behavior, and at testing we excluded those intruders that displayed agonistic behavior. All testing sessions were digitally recorded and the behavior of the subject was quantified using Noldus Observer. We quantified the total duration of the following categories of behavior: submissive/defensive (flee, avoid, upright and side defensive postures, tail-up, stretch-attend, head flag); aggressive (chase, attack including bite, upright and side offensive postures); social (nose touching, sniff, approach); and nonsocial (locomotion, grooming, nesting, feeding) (Albers et al., 2002). We also quantified the frequency of flees, stretch-attend postures, and attacks. All video scoring was done by a single researcher blind to experimental conditions. On a subset of videos, inter-rater reliability in submissive/defensive behavior was > 90%.
2.4 Drugs
Flesinoxan-hydrochloride (courtesy of Solvay Pharmaceuticals, now part of Abbot Laboratories) was dissolved in sterile saline (pH = 6.1), which was used as a vehicle control at a similar pH. Flesinoxan precipitates at physiological pH and is commonly used at pH ranging from 4.2–5.5 (Compaan et al., 1997; Cooper et al., 2008; Sibug et al., 2000; Sporton et al., 1991). Flesinoxan is a highly selective 5-HT1A receptor agonist (Schoeffter and Hoyer, 1988). WAY-100635 (Sigma Aldrich) was also dissolved in sterile saline (pH = 7.4). WAY-100635 is a highly selective antagonist for the 5-HT1A receptor (Mos et al., 1997). Flesinoxan, WAY-100635, and saline vehicle were injected at volumes of 200 nl per side.
2.5 Experiments 1 and 2: 5-HT1A receptor agonist and conditioned defeat
We designed Experiment 1 to test whether injection of a 5-HT1A receptor agonist into the BLA prior to social defeat would decrease the acquisition of conditioned defeat. We bilaterally infused flesinoxan (400 ng, N = 8; 800 ng, N = 11; or 1200 ng, N = 10) or vehicle (N = 11) into the BLA 10 min prior to a 15 min social defeat. For no defeat control subjects, we bilaterally infused flesinoxan (1200 ng, N = 9) or vehicle (N = 10) into the BLA 10 min prior to a 15 min exposure to an empty RA cage. The doses of flesinoxan used here are based on doses that were effective in the hamster DRN (Cooper et al., 2008). For drug injection, a 1 µl syringe (Harvard Instruments) was connected to an injection needle via PE-20 polyethylene tubing. Injections took place over a 1 min period using a Harvard Syringe Pump (Harvard Instruments), and needles were left in place for 1 min after the infusion to allow drug diffusion. Any animal that did not receive successful bilateral injections was excluded from data analysis. Subjects were tested for conditioned defeat 24 h following social defeat.
Experiment 2 was designed to test whether injection of a 5-HT1A receptor agonist into the BLA prior to behavioral testing would decrease the expression of conditioned defeat. Subjects received a 15 min social defeat or empty RA cage exposure. Twenty-four hours later, we bilaterally infused flesinoxan (400 ng, N = 12; 800 ng, N = 10; or 1200 ng, N = 11) or vehicle (N = 11) into the BLA 10 min prior to behavioral testing. For no defeat control subjects, we bilaterally infused flesinoxan (1200 ng, N =7) or vehicle (N = 9) into the BLA 10 min prior to behavioral testing.
2.6 Experiments 3 and 4: 5-HT1A receptor antagonist and conditioned defeat
In Experiment 3, we tested whether injection of a 5-HT1A receptor antagonist into the BLA prior to social defeat would increase the acquisition of conditioned defeat. We bilaterally infused WAY-100635 (400 ng, N = 10 or 1600 ng, N = 11) or vehicle (N = 10) into the BLA 10 min prior to a 5 min social defeat. For no defeat control subjects, we bilaterally infused WAY-100635 (400 ng, N = 12) or vehicle (N = 10) into the BLA 10 min prior to a 5 min empty RA cage exposure. We selected these doses of WAY-100635 on the basis of previous research in which we injected WAY-100635 into the DRN (Cooper et al., 2008). Twenty-four hours after social defeat, we tested subjects for conditioned defeat.
In Experiment 4, we tested whether injection of a 5-HT1A receptor antagonist into the BLA prior to behavioral testing would increase the expression of conditioned defeat. Subjects received a 5 min social defeat or empty cage exposure. Twenty-four hours later, we bilaterally infused WAY-100635 (400 ng, N = 10 or 1600 ng, N = 10) or vehicle (N = 10) into the BLA 10 min prior to behavioral testing. For no defeat control subjects, we bilaterally infused WAY-100635 (400 ng, N = 11) or vehicle (N = 13) into the BLA 10 min prior to testing.
2.7 Histology
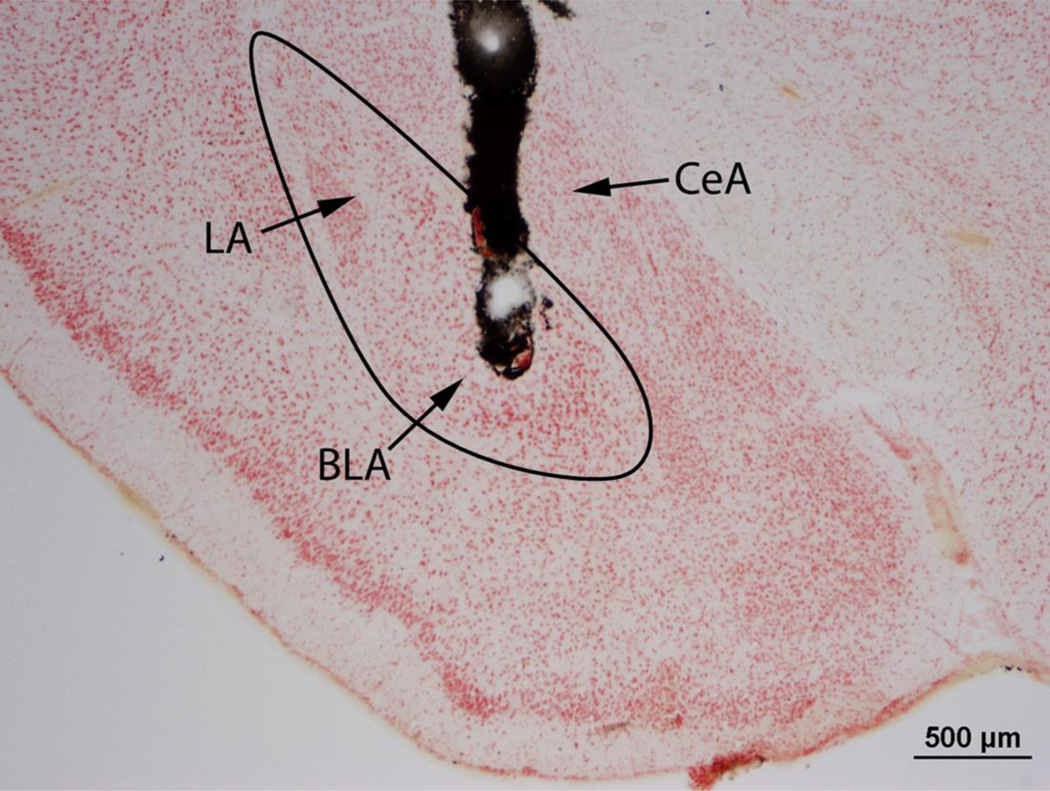
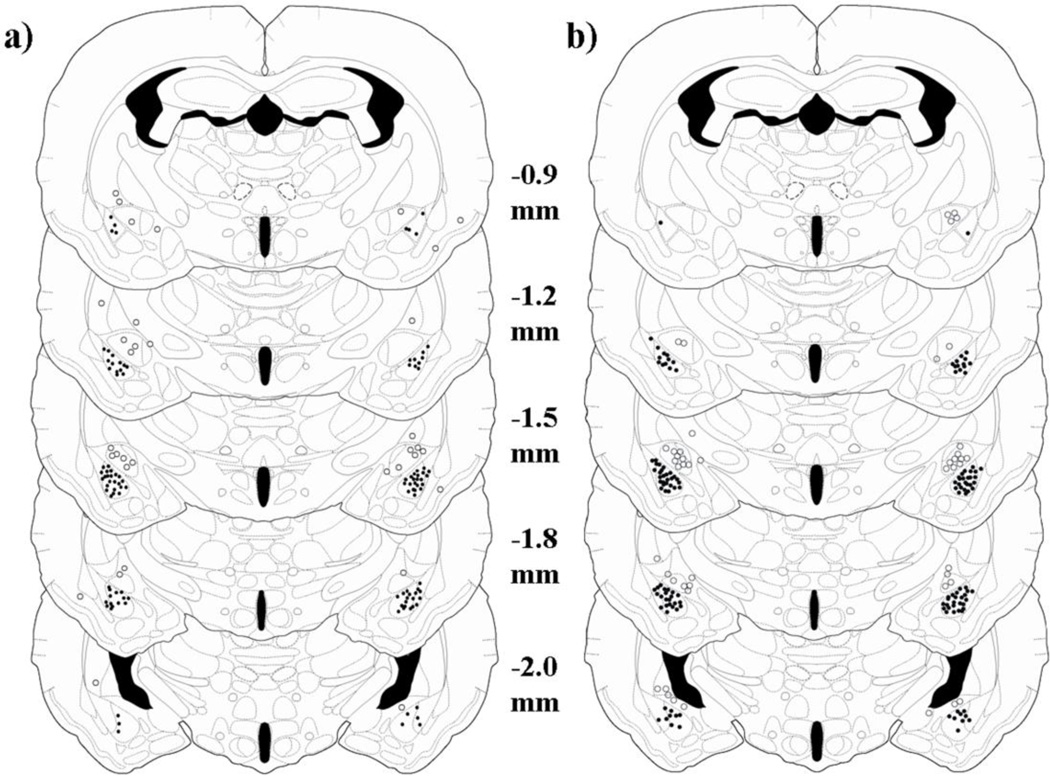
Immediately following testing, animals were given a lethal cocktail of 93% sodium pentobarbital and 7% isopropyl alcohol (Sleep Away, Webster Veterinary) and infused with 200 nl of India ink into the BLA. Brains were removed, frozen on dry ice, and stored at −80° C. Brains were sliced at 30 µm on a cryostat, and sections were stained with neutral red and coverslipped. Sections were examined under a light microscope for evidence of ink in the BLA (Fig. 1). Subjects with bilateral injection sites within 100 µm of the BLA were included in analysis (Fig. 2). Subjects with bilateral injection sites >300 µm from the BLA were analyzed as anatomical controls. We excluded subjects with a unilateral injection site > 100 µm from the BLA and one subject with bilateral injection sites that were on the border of the BLA (100–300 µm).
Fig. 1.
A representative photomicrograph is shown of a hamster coronal brain section injected with India ink and stained with neutral red. The injection site is clearly visible within the BLA. The basolateral complex (BLA and LA) is roughly outlined. BLA – basolateral amygdala, LA – lateral amygdala, CeA – central amygdala.
Fig. 2.
The location of BLA injection sites is shown using illustrations adapted from a hamster stereotaxic atlas (Morin and Wood, 2001). The distances shown for each illustration are relative to bregma. A schematic shows injection sites for a) Experiments 1 and 2 and b) Experiments 3 and 4. Black circles indicate the approximate placement of injection sites within the BLA. Open circles represent injection sites for anatomical controls. Misplaced injections were most often given into the central amygdala, but also occurred in the piriform cortex, caudate putamen, and globus pallidus.
2.8 Data Analysis
Conditioned defeat data were analyzed using a 2-way between subjects analysis of variance (ANOVA) with one factor as defeat experience (defeat or no defeat control) and the second factor as dose of drug. The duration of submissive/defensive, aggressive, social, and nonsocial behaviors were used as dependent variables. Agonistic behavior of the RAs during social defeat was analyzed using 1-way ANOVAs. Statistically significant differences found in the 2-way ANOVA were further analyzed using either a 1-way ANOVA for defeated subjects with Tukey’s post hoc tests or an independent sample t-test for no defeat controls. All statistical tests were two-tailed, and the level was set at p ≤ 0.05.
3. Results
3.1 Experiment 1: 5-HT1A receptor agonist infused into the BLA at acquisition
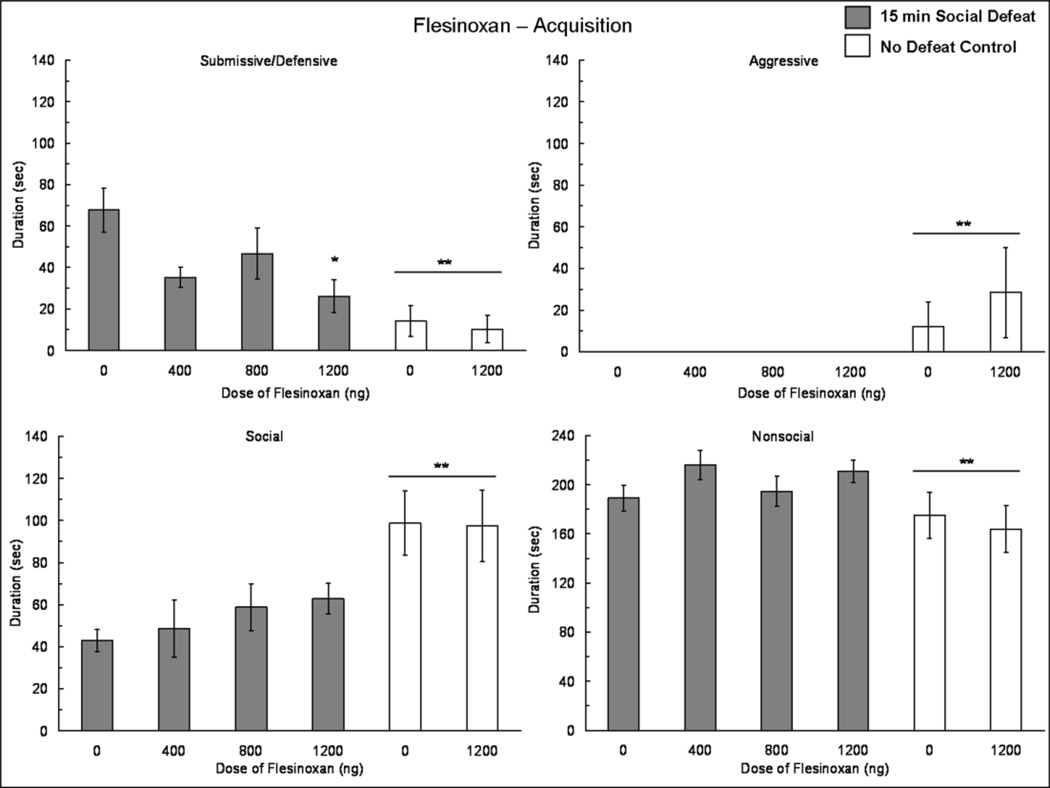
The injection of flesinoxan into the BLA prior to social defeat reduced the acquisition of conditioned defeat (Fig. 3). We found a significant main effect of defeat experience (F(1,53) = 14.38, p < 0.001) and a significant defeat experience × dose of flesinoxan interaction (F(1,53) = 4.23, p = 0.045) on the total duration of submissive/defensive behavior displayed at testing. Specifically, defeated individuals injected with 1200 ng of flesinoxan displayed a lower duration of submissive/defensive behavior at testing when compared to defeated vehicle controls (F(3,36) = 3.31, p = 0.031; Tukey, p = 0.024). Defeated animals injected with 1200 ng of flesinoxan did not significantly differ from any no defeat control group in submissive/defensive behavior.
Fig. 3.
Durations (mean ± SE) of submissive and defensive, aggressive, social and nonsocial behaviors are shown during a 5 min test with a non-aggressive intruder. Social defeat animals (shaded bars) received an injection of flesinoxan or vehicle into the BLA 10 min prior to 15 min social defeat training. No defeat controls (white bars) received an injection of flesinoxan or vehicle into the BLA 10 min prior to exposure to the empty cage of a resident aggressor. Asterisks (*) indicate treatments that differ from socially defeated vehicle controls. Double asterisks (**) positioned above a horizontal line indicate that defeated individuals differ from no defeat controls.
We found a main effect of defeat experience on aggressive (F(1,53) = 4.65, p = 0.036), social (F(1,53) = 14.54, p < 0.001), and nonsocial (F(1,53) = 4.85, p = 0.032) behavior displayed at testing (Fig. 3). We did not find a defeat experience × dose of flesinoxan interaction for aggressive, social or nonsocial behavior displayed at testing. Additionally, we did not find a drug effect in no defeat control groups for any category of behavior.
Fifteen animals received injections that were > 300 µm outside of the BLA and were analyzed as anatomical controls. Flesinoxan infused outside of the BLA prior to social defeat did not appear to reduce submissive/defensive behavior at testing (Vehicle: 35.26 ± 26.05, N = 4; 400 ng: 36.03 ± 22.85, N = 2; 800 ng: 42.55 ± 8.43, N = 3; 1200 ng: 29.66 ± 10.05, N = 6).
To ensure that the effect of flesinoxan on the acquisition of conditioned defeat was not due to differences in the quality of social defeat, we scored the behavior of the RAs during social defeat. The treatment groups did not significantly differ in any measure (Table 1).
Table 1.
Flesinoxan treatment did not alter social defeat experience (Mean ± SE)
| Vehicle | 400 ng | 800 ng | 1200 ng | p | |
|---|---|---|---|---|---|
| Aggression (sec) | 389.8 ± 45.8 | 415.5 ± 71.1 | 424.7 ± 62.9 | 354.4 ± 41.2 | ns |
| Latency to Attack (sec) | 76.2 ± 31.7 | 99.7 ± 57.4 | 79.7 ± 32.8 | 64.8 ± 42.9 | ns |
| Number of Attacks | 20.4 ± 3.1 | 19.2 ± 2.7 | 17.4 ± 1.7 | 15.1 ± 2.8 | ns |
Subjects received injection of flesinoxan (400 ng, 800 ng, or 1200 ng) or vehicle into the basolateral amygdala 10 min prior to social defeat training. ns = not significant
3.2 Experiment 2: 5-HT1A receptor agonist infused into the BLA at expression
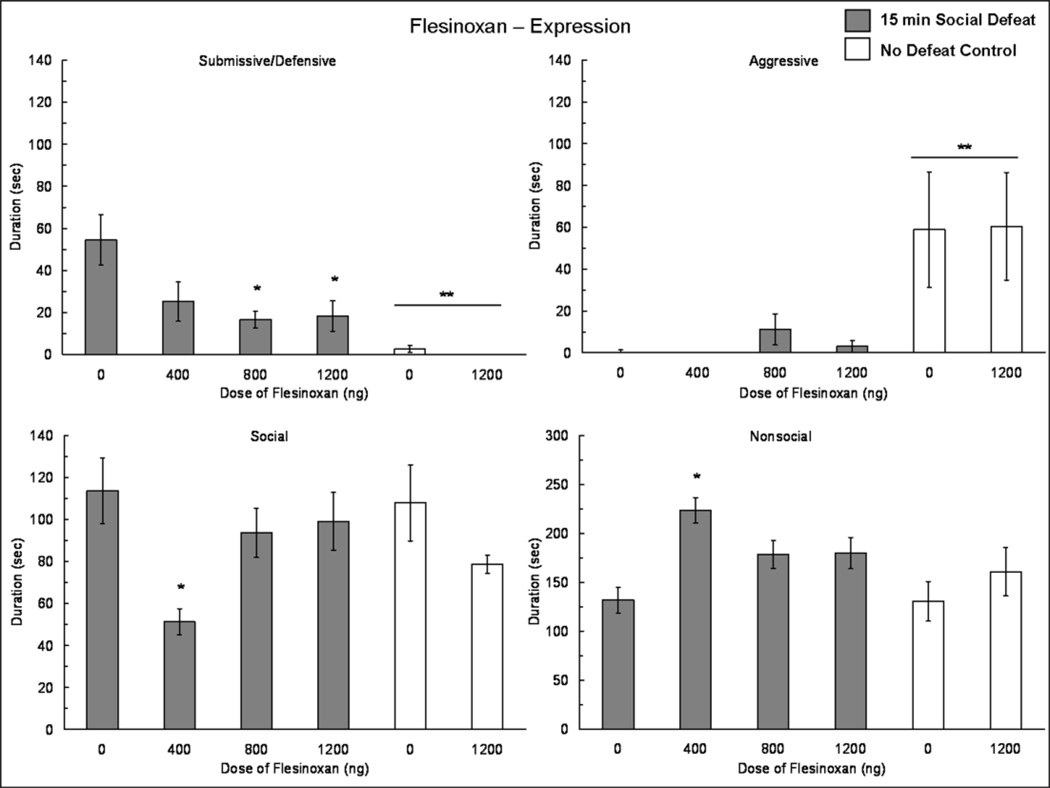
The injection of flesinoxan into the BLA prior to behavioral testing reduced the expression of conditioned defeat (Fig. 4). We found a significant main effect of defeat experience (F(1,53) = 17.63, p < 0.001) and a significant defeat experience × dose of flesinoxan interaction (F(1,53) = 4.00, p = 0.05) on the total duration of submissive/defensive behavior displayed at testing. Specifically, defeated individuals injected with 800 ng and 1200 ng of flesinoxan displayed a lower duration of submissive/defensive behavior at testing when compared to vehicle controls (F(3,40) = 3.87, p = 0.016; Tukey, p = 0.027 and; Tukey, p = 0.031, respectively). There was no significant difference in submissive/defensive behavior between defeated individuals injected with 800 ng or 1200 ng of flesinoxan and vehicle or drug-treated no defeat control individuals. Among no defeat controls, there was no effect of flesinoxan on the duration of submissive/defensive behavior at testing. There was a main effect of defeat experience on aggressive behavior at testing (F(1,54) = 21.32, p < 0.001), indicating that no defeat controls showed significantly more aggressive behavior at testing than did defeated animals (Fig. 4). There was not a significant defeat experience × dose of flesinoxan interaction on aggression.
Fig. 4.
Durations (mean ± SE) of submissive and defensive, aggressive, social and nonsocial behaviors are shown during a 5 min test with a non-aggressive intruder. Animals that experienced a 15 min social defeat (shaded bars) and no defeat controls (white bars) received an injection of flesinoxan or vehicle into the BLA 10 min prior to behavioral testing. Asterisks (*) indicate treatments that differ from socially defeated vehicle controls. Double asterisks (**) positioned above a horizontal line indicate that defeated individuals differ from no defeat controls.
There was no main effect of defeat experience and no interaction of defeat experience × dose of flesinoxan on the amount of social behavior displayed at testing, although there was a significant main effect of dose (F(3,54) = 5.79, p = 0.002). Further analysis indicated there was a significant difference among defeated animals (F(3,40) = 5.075, p = 0.005), such that injection of 400 ng of flesinoxan resulted in a lower duration of social behavior compared to vehicle controls (Tukey, p = 0.004). There was no effect of flesinoxan on the duration of social behavior among no defeat controls. A similar pattern was observed for nonsocial behavior, such that there was no main effect of defeat experience, no defeat experience × flesinoxan interaction, and a significant main effect of dose (F(1,54) = 5.98, p = 0.001). Among defeated animals, injection of 400 ng of flesinoxan into the BLA resulted in a greater duration of nonsocial behavior when compared to vehicle animals (F(3,40) = 7.29, p = 0.001; Tukey, p < 0.001). There was no effect of flesinoxan dose on the duration of nonsocial behavior in no defeat controls. Although 400 ng of flesinoxan disrupted the balance of social and nonsocial behavior, the elevated nonsocial behavior was not due to hyperlocomotion and likely did not contribute to impairment in conditioned defeat because higher doses of flesinoxan did not produce similar disruption.
Ten animals received injections that were more than 300 µm outside of the BLA and were analyzed as anatomical controls. Flesinoxan infused outside of the BLA prior to behavioral testing did not appear to reduce submissive/defensive behavior at testing (Vehicle: 79.1 ± 0.0, N = 1; 400 ng: 21.5 ± 0.0, N = 1; 800 ng: 31.25 ± 14.43, N = 4; 1200 ng: 79.84 ± 31.49, N = 4). To increase the sample size of our anatomical control groups, we combined the vehicle, 800 ng and 1200 ng groups from experiments 1 and 2. Analysis of the combined groups suggests that flesinoxan infused outside of the BLA either prior to social defeat or to behavioral testing did not significantly reduce submissive/defensive behavior at testing (p > 0.05; Vehicle: 44.03 ± 22.01, N = 5; 800 ng: 36.1 ± 8.66, N = 7; 1200 ng: 53.1 ± 16.65, N = 9).
3.3 Experiment 3: 5-HT1A receptor antagonist infused into the BLA at acquisition
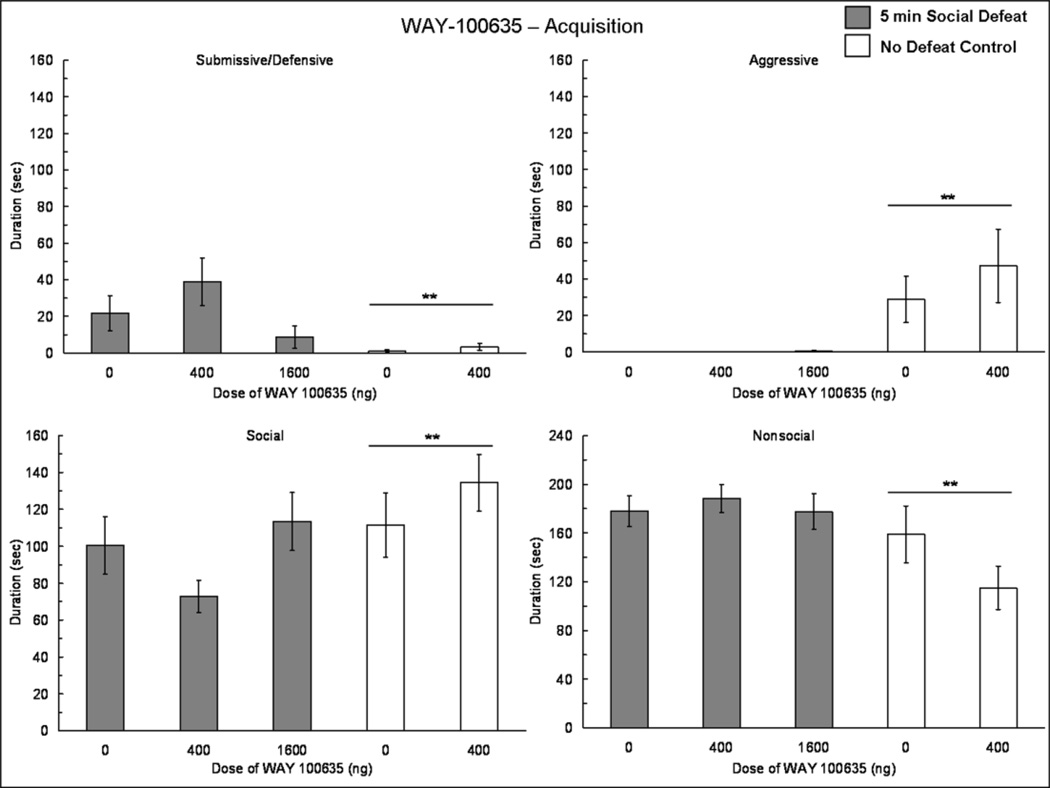
Injecting WAY-100635 into the BLA prior to social defeat did not increase the acquisition of conditioned defeat (Fig. 5). We found a significant main effect of defeat experience on submissive/defensive behavior (F(1,48) = 13.80, p = 0.001), indicating that defeated animals showed significantly more submissive/defensive behavior at testing than did no defeat controls (Fig. 5). We found a main effect of defeat experience on aggressive (F(1,48) = 10.64, p = 0.002), social (F(1,48) = 5.81, p = 0.02), and nonsocial (F(1,48) = 7.57, p = 0.008) behavior displayed at testing (Fig. 5). No other significant differences were found.
Fig. 5.
Durations (mean ± SE) of submissive and defensive, aggressive, social and nonsocial behaviors are shown during a 5 min test with a non-aggressive intruder. Social defeat animals (shaded bars) received an injection of WAY-100635 or vehicle into the BLA 10 min prior to 5 min social defeat training. No defeat controls (white bars) received an injection of WAY-100635 or vehicle into the BLA 10 min prior to exposure to the empty cage of a resident aggressor. Double asterisks (**) positioned above a horizontal line indicate that defeated individuals differ from no defeat controls.
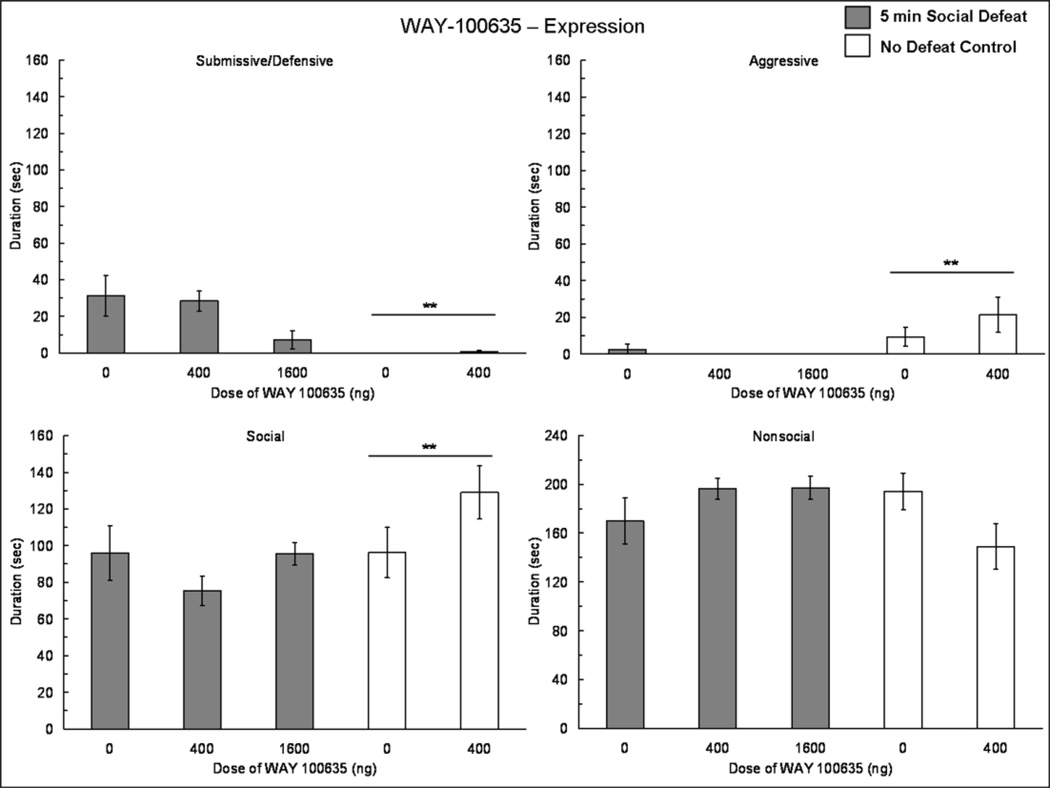
3.4 Experiment 4: 5-HT1A receptor antagonist infused into the BLA at expression
The injection of WAY-100635 into the BLA prior to behavioral testing did not increase the expression of conditioned defeat (Fig. 6). There was a significant main effect of defeat experience on submissive/defensive behavior (F(1,49) = 29.30, p < 0.001), such that socially defeated animals displayed more submissive/defensive behavior than did no defeat controls (Fig. 6). We found a main effect of defeat experience on aggressive (F(1,49) = 7.16, p = 0.01) and social (F(1,49) = 4.72, p = 0.035) behavior displayed at testing (Fig. 6). No other significant differences were found.
Fig. 6.
Durations (mean ± SE) of submissive and defensive, aggressive, social and nonsocial behaviors are shown during a 5 min test with a non-aggressive intruder. Animals that experienced a 5 min social defeat (shaded bars) and no defeat controls (white bars) received an injection of WAY-100635 or vehicle into the BLA 10 min prior to behavioral testing. Double asterisks (**) positioned above a horizontal line indicate that defeated individuals differ from no defeat controls.
4. Discussion
We have shown that injection of the 5-HT1A receptor agonist flesinoxan into the BLA decreases both the acquisition and expression of conditioned defeat. These results suggest that pharmacological activation of 5-HT1A receptors in the BLA prior to social defeat training is sufficient to impair the formation of conditioned defeat, and that their activation prior to testing is sufficient to disrupt the production of submissive and defensive behavior. The effects of flesinoxan injection appear to be specific to the BLA, as injections of flesinoxan outside the BLA had no effect. Additionally, flesinoxan treatment did not alter the behavior of no defeat control animals, which indicates that activation of BLA 5-HT1A receptors specifically modulates defeat-induced behavioral changes. We found that activation of BLA 5-HT1A receptors modulates defeat-induced changes in the duration of submissive and defensive, but not aggressive, behavior. These results are consistent with previous research showing that changes in submissive and defensive, but not aggressive, behavior are controlled by fear and anxiety-related neural circuitry in the amygdala (Jasnow et al., 2004; Jasnow et al., 2005; Markham and Huhman, 2008). We also found that injection of the 5-HT1A receptor antagonist WAY-100635 into the BLA did not alter the acquisition or expression of conditioned defeat. Together our results suggest that activation of BLA 5-HT1A receptors disrupts the acquisition and expression of conditioned defeat whereas blockade of BLA 5-HT1A receptors does not alter conditioned defeat.
There has been great interest in the role of 5-HT1A receptors in the expression of depression- and anxiety-related behavior. Overall, increased neural signaling at 5-HT1A receptors is associated with reduced anxiety. For example, 5-HT1A receptor partial agonists such as buspirone are used clinically for their anxiolytic action (Hindmarch et al., 1992; Traber and Glaser, 1987). Neuroimaging studies have shown that individuals with lower 5-HT1A binding are more likely to display clinical levels of anxiety and have increased basal cortisol levels (Lanzenberger et al., 2007; Lanzenberger et al., 2010; Neumeister et al., 2004; Rabiner et al., 2002; Tauscher et al., 2001). In animal studies, 5-HT1A knockout mice display significantly higher levels of anxiety-like behavior compared to control animals (Akimova et al., 2009; Lesch, 2001; Ramboz et al., 1998). Additionally, transgenic mice that overexpress the 5-HT1A receptor show decreased anxiety-like behavior when compared with wild type mice (Kusserow et al., 2004).
The anxiolytic effect of systemic 5-HT1A receptor treatments may be mediated by somatodendritic autoreceptors in the DRN or postsynaptic receptors in the forebrain. At least part of the anxiolytic action of 5-HT1A receptor activation is mediated by inhibitory postsynaptic receptors in several forebrain regions (Kia et al., 1996; Pazos and Palacios, 1985). For example, pharmacological activation of 5-HT1A receptors in the hippocampus reduces fear and anxiety-like behavior in several paradigms, including fear conditioning (Li et al., 2006; Stiedl et al., 2000), elevated plus maze (Zhang et al., 2010), and novelty suppressed feeding (Zhang et al., 2010). The BLA is another important site where 5-HT1A receptor activation can modulate fear-related and anxiety-like behavior. Injection of a selective 5-HT1A receptor agonist into the BLA reduces fear conditioning (Li et al., 2006), fear-potentiated startle (Groenink et al., 2000), and inhibitory avoidance in the elevated T-maze (Zangrossi et al., 1999). These results are consistent with our current findings and together suggest that pharmacological activation of BLA 5-HT1A receptors attenuates fear and anxiety when responses are conditioned. In contrast, pharmacological activation of BLA 5-HT1A receptors increases fear and anxiety when responses are unconditioned, such as in social interaction tests (Gonzalez et al., 1996) and for escape behavior in the elevated T-maze (Zangrossi et al., 1999). It might be that conditioned and unconditioned emotional responses are differentially affected by activation of BLA 5-HT1A receptors.
5-HT1A receptor antagonists such as WAY-100635 have been used to block the behavioral effects of 5-HT1A receptor activation (File et al., 1996; Gonzalez et al., 1996). Also, some researchers have used WAY-100635 for its ability to act as a silent antagonist, indicating that there was no expected effect of WAY-100635 when administered alone (File et al., 1996; Stiedl et al., 2000). Blocking 5-HT1A receptors by themselves has produced inconsistent effects on anxiety-related behavior and passive avoidance. Injection of WAY-100635 into the dorsal periaqueductal gray failed to alter fear conditioning, although injection of the 5-HT1A receptor agonist 8-OH-DPAT decreased it (Broiz et al., 2008). In contrast, injection of WAY-100635 into the ventral hippocampus was shown to reduce anxiety-like behavior in the elevated plus maze (Nunes-de-Souza et al., 2002), and systemic administration of WAY-100635 has been shown to enhance passive avoidance learning (Madjid et al., 2006). Site-specific injections of WAY-100635 are often given at much higher doses (3000 ng or greater) than those used in our study (400–1600 ng). We selected doses of WAY-100635 based on our previous research, and the work of others, showing behavioral effects of WAY-100635 when injected into the DRN (Cooper et al., 2008; Pobbe and Zangrossi, 2005). It is possible that higher doses of WAY-100635 are required to fully block the post-synaptic 5-HT1A receptors that occur outside of the DRN. In sum, our findings with WAY-100635 suggest the serotonergic activity at BLA 5-HT1A receptors is not necessary for the acquisition and expression of conditioned defeat, because conditioned defeat occurs normally without it.
Although 5-HT1A receptors modulate the expression of anxiety-like behavior following stressful events (Youn et al., 2009), much less is known about the role of BLA 5-HT1A receptors in the acquisition of aversive memories, including conditioned fear. Although the BLA is a critical brain region controlling cued fear conditioning, data on 5-HT1A receptor modulation of fear conditioning are limited to the hippocampus. Stiedl et al. (2000) found that bilateral intrahippocampal injection of 8-OH-DPAT prior to the training phase of fear conditioning resulted in decreased freezing to both context and cue 24 h later. Pretreatment with both subcutaneous and intrahippocampal WAY-100635 completely reversed the effect on contextual freezing but only partially reversed cued freezing (Stiedl et al., 2000). In a separate study, 8-OH-DPAT given peripherally prior to training in a passive avoidance paradigm resulted in decreased retention of avoidance (Misane et al., 1998). These studies indicate that activation of forebrain 5-HT1A receptors impairs the formation of conditioned fear. Our results suggest that activation of 5-HTA receptors in the BLA impairs the acquisition of stress-related changes in behavior. Our results are consistent with previous studies that indicate that the BLA is the primary site of neural plasticity controlling the formation of conditioned defeat (Day et al., 2011; Jasnow et al., 2005; Markham et al., 2010). Specifically, NMDA receptors in the BLA are a critical component of the neurochemical signals controlling the formation of conditioned defeat (Day et al., 2011; Jasnow et al., 2004). Others have suggested that 5-HT1A receptors interact with glutamatergic and cholinergic systems in the frontal cortex and hippocampus to alter learning and memory processes (Kehr et al., 2010; Madjid et al., 2006; Ogren et al., 2008). Our results suggest that the conditioned defeat model may provide a valuable approach for investigating 5-HT1A receptor modulation of neural processes in the BLA that underlie memories for aversive events. One interesting possibility is that 5-HT1A receptors activation impairs the formation of conditioned defeat by modulating NMDA receptor-dependent mechanisms in the BLA. In sum, activation of 5-HT1A receptors in the hippocampus appears to disrupt the formation of conditioned fear, whereas 5-HT1A receptors in the BLA appear more critical for conditioned defeat.
In conclusion, we have found that injection of flesinoxan into the BLA reduced both the acquisition and expression of conditioned defeat. These results indicate that the formation of conditioned defeat and the display of submissive and defensive behavior at testing can be reduced by activation of BLA 5-HT1A receptors. This finding extends our previous research on the role of 5-HT in conditioned defeat. We have shown previously that flesinoxan injection in the DRN blocks both the acquisition and expression of conditioned defeat, and injection of WAY-100635 enhances both acquisition and expression (Cooper et al., 2008). 5-HT1A receptors in the DRN are autoreceptors, and their activation has been shown to decrease the release of 5-HT in DRN projection regions (Sharp et al., 1989). From our DRN study, we concluded that 5-HT release in DRN projection regions enhances the formation and display of conditioned defeat behavior. The current work expands upon the DRN findings by beginning to explore the mechanisms of 5-HT action in the BLA, a key neural structure underlying the plasticity and behavioral output associated with conditioned defeat (Day et al., 2011). Although we found that activation of BLA 5-HT1A receptors reduces the acquisition and expression of conditioned defeat, we also found that blockade of BLA 5-HT1A receptors has no effect on conditioned defeat. These results suggest that activation of BLA 5-HT1A receptors is sufficient to impair conditioned defeat, although there appears to be a limited role for endogenous 5-HT activity at BLA 5-HT1A receptors. We expect that endogenous 5-HT may act at other 5-HT receptors, such as 5-HT2 receptors, to enhance the acquisition and expression of conditioned defeat. Others have shown that activation of 5-HT2 receptors facilitates eyeblink conditioning (Harvey, 2003), the expression of learned helplessness (Strong et al., 2009), and anxiety in an open field test (Campbell and Merchant, 2003). Also, recent evidence suggests that serotonergic modulation of the BLA pyramidal neurons is largely controlled by 5-HT2A receptor activity (Jiang et al., 2009). Future work will need to address the mechanisms by which 5-HT can act at multiple receptors, and perhaps in multiple brain regions, to modulate the acquisition and expression of conditioned defeat.
Research Highlights.
Activation of 5-HT1A receptors in the BLA impairs conditioned defeat.
5-HT1A receptors in the BLA modulate the display of stress-induced behavior.
5-HT1A receptors in the BLA modulate the formation of stress-related memories.
Acknowledgements
This work was supported by National Institutes of Health grant R21 MH085230 to MAC. We thank our team of undergraduate students for their technical assistance, including Daniel Curry, Travis Goode, and Cody Swallows.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Albers HE, Huhman KL, Meisel RL. Hormonal basis of social conflict and communication. In: Pfaff DW, et al., editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 393–433. [Google Scholar]
- Almada RC, Borelli KG, Albrechet-Souza L, Brandao ML. Serotonergic mechanisms of the median raphe nucleus-dorsal hippocampus in conditioned fear: output circuit involves the prefrontal cortex and amygdala. Behav Brain Res. 2009;203:279–287. doi: 10.1016/j.bbr.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience. 1998;82:147–159. doi: 10.1016/s0306-4522(97)00282-0. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen BS, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Broiz AC, Oliveira LC, Brandao ML. Regulation of conditioned and unconditioned fear in rats by 5-HT1A receptors in the dorsal periacqueductal gray. Pharmacol Biochem Behav. 2008;89:76–84. doi: 10.1016/j.pbb.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce actue fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Campeau S, Miserendino MJ, Davis M. Intra-amygdala infustion of the N-methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- Compaan JC, Groenink L, Van der Gugten J, Maes RAA, Olivier B. Pretreatment with 5-HT1A receptor agonist flesinoxan attenuates Fos protein in rat hypothalamus. Eur J Pharmacol. 1997;324:161–168. doi: 10.1016/s0014-2999(97)00071-x. [DOI] [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Cassella JV, Kehne JH. Serotonin does not mediate anxiolytic effects of buspirone in the fear-potentiated startle paradigm: comparison with 8-OH-DPAT and isapirone. Psychopharmacology (Berl) 1988;94:14–20. doi: 10.1007/BF00735873. [DOI] [PubMed] [Google Scholar]
- Dawson L, Watson J. Vilazodone: a 5-HT1A receptor agonist/serotonin transporter inhibitor for the treatment of affective disorders. CNS Neurosci Ther. 2009;15:107–117. doi: 10.1111/j.1755-5949.2008.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DE, Cooper MA, Markham CM, Huhman KL. NR2B subunit of the NMDA receptor in the basolateral amygdala is necessary for the acquisition of conditioned defeat in Syrian hamsters. Behav Brain Res. 2011;217:55–59. doi: 10.1016/j.bbr.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Kim J. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht H-R, Siegfried B, Waser PG. Learning of submissive behavior in mice: a new model. Behav Processes. 1982;7:235–245. doi: 10.1016/0376-6357(82)90038-9. [DOI] [PubMed] [Google Scholar]
- Gerwitz J, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature. 1997;388:471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- Groenink L, Joordens RJ, Hijzen TH, Dirks A, Olivier B. Infusion of flesinoxan into the amygdala blocks the fear-potentiated startle. Neuroreport. 2000;11:2285–2288. doi: 10.1097/00001756-200007140-00043. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Naciti C, Brand L, Stein DJ. Serotonin and stress: protective or malevolent actions in the biobehavioral response to repeated trauma? Ann N Y Acad Sci. 2004;1032:267–272. doi: 10.1196/annals.1314.035. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Role of the serotonin 5-HT(2A) receptor in learning. Learn Mem. 2003;10:355–362. doi: 10.1101/lm.60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neuroiology of mood and anxiety disorders: precilincal and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heinrichs S, Pich E, Miczek KA, Britton K, Koob G. Corticotropin-releasing factor antagonist redues emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;58:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Schillingford J, Kerr JS, Hesselink JM. The comparative psychopharmacology of 5-HT1A agonists. In: Stahl SM, et al., editors. Serotonin 1A Receptors in Depression and Anxiety. New York: Raven Press; 1992. pp. 109–117. [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Moore TO, Mougey EH, Meyerhoff JL. Hormonal responses to fighting in hamsters: separation of physical and psychological causes. Physiol Behav. 1992;51:1083–1086. doi: 10.1016/0031-9384(92)90097-l. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Cooper MA, Huhman KL. N-methyl-D-aspartate receptors in the amygdala are necessary for the acquisition and expression of conditioned defeat. Neuroscience. 2004;123:625–634. doi: 10.1016/j.neuroscience.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci. 2005;119:1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology. 2009;34:410–423. doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- Josselyn S, Shi C, Carlezon W, Jr, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney A, Jessop D, Harbuz M, Marsden CA, Hogg S, Blackburn-Munro R. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kehr J, Hu XJ, Yoshitake T, Wang FH, Osborne P, Stenfors C, Ogren SO. The selective 5-HT(1A) receptor antagonist NAD-299 increases acetylcholine release but not extracellular glutamate levels in the frontal cortex and hippocampus of awake rat. Eur Neuropsychopharmacol. 2010;20:487–500. doi: 10.1016/j.euroneuro.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S, Resiter R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kuo C-J, Tang H-S, Tsay C-J, Hu W-H, Chen C-C. Prevalence of psychiatric disorders among bereaved survivors of a disastrous earthquake in Taiwan. Psychiatr Serv. 2003;54:249–251. doi: 10.1176/appi.ps.54.2.249. [DOI] [PubMed] [Google Scholar]
- Kusserow H, Davies B, Hortnagl H, Ingo V, Stroh T, Bert B, Deng DR, Fink H, Veh RW, Theuring F. Reduced anxiety-related behaviour in transgenic mice overexpression serotonin(1A) receptors. Brain Res Molec Brain Res. 2004;129:104–116. doi: 10.1016/j.molbrainres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Lanzenberger R, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Lanzenberger R, Wadsak W, Spindelegger C, Mitterhauser M, Akimova E, Mien L, Fink M, Moser U, Savli M, Kranz GS, Hahn A, Kletter K, Kasper S. Cortisol plasma levels in social anxiety disorder patients correlate with serotonin-1A receptor binding in limbic brain regions. Int J Neuropsychopharmacol. 2010;13:1129–1143. doi: 10.1017/S1461145710000581. [DOI] [PubMed] [Google Scholar]
- Lesch K. Mouse anxiety: the power of knockout. Pharmacogenomics J. 2001;1:187–192. doi: 10.1038/sj.tpj.6500016. [DOI] [PubMed] [Google Scholar]
- Li X, Inoue T, Abekawa T, Weng S, Nakagawa S, Izumi T, Koyama T. 5-HT1A receptor agonist affects fear conditioning through stimulations of the postsynaptic 5-HT1A receptors in the hippocampus and amygdala. Eur J Pharmacol. 2006;532:74–80. doi: 10.1016/j.ejphar.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Madjid N, Tottie EE, Luttgen M, Meister B, Sandin J, Kuzmin A, Stiedl O, Ogren SO. 5-Hydroxytryptamine 1A receptor blockade facilitates aversive learning in mice: interactions with cholinergic and glutamatergic mechanisms. J Pharmacol Exp Ther. 2006;316:581–591. doi: 10.1124/jpet.105.092262. [DOI] [PubMed] [Google Scholar]
- Markham CA, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem. 2010;17:109–116. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Davis M. Effects of septal lesions on fear-potentiated startle, and on the anxiolytic effects of buspirone and diazepam. Physiol Behav. 1991;49:603–611. doi: 10.1016/0031-9384(91)90286-w. [DOI] [PubMed] [Google Scholar]
- Misane I, Johansson C, Ogren SO. Analysis of the 5-HT1A receptor involvement in passive avoidance in the rat. Br J Pharmacol. 1998;125:499–509. doi: 10.1038/sj.bjp.0702098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. San Diego: Academic Press; 2001. [Google Scholar]
- Mos J, Van Hest A, Van Drimmelen M, Herremans AH, Olivier B. The putative 5-HT1A receptor antagonist DU125530 blocks the discriminative stimulus of the 5-HT1A receptor agonist flesinoxan in pigeons. Eur J Pharmacol. 1997;325:145–153. doi: 10.1016/s0014-2999(97)00131-3. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-de-Souza RL, Canto-de-Souza A, Rodgers RJ. Effects of intra-hippocampal infusion of WAY-100635 on plus-maze behavior in mice. Influence of site of injection and prior test experience. Brain Res. 2002;927:87–96. doi: 10.1016/s0006-8993(01)03335-2. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekstrom JC, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5-HT1A receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios J. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 Receptors. Brain Res. 1985;364:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Zangrossi HJ. 5-HT(1A) and 5-HT(2A) receptors in the rat dorsal periaqueductal gray mediate the antipanic-like effect induced by the stimulation of serotonergic neurons in the dorsal raphe nucleus. Psychopharmacology (Berl) 2005;183:314–321. doi: 10.1007/s00213-005-0196-z. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Messa C, Sarget PA, Husted-Kjaer K, Montgomery A, Lawrence AD, Bench CJ, Gunn RN, Cowen P, Grasby PM. A database of [(11C)]WAY-100635 binding to 5-HT(1A) recpetors in normal male volunteers: normative data and relationship to methodological, dmeographic, physiological, and behavioral variables. Neuroimage. 2002;15:620–632. doi: 10.1006/nimg.2001.0984. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Brodkin JD, Geyer MA. GABA-A and 5-HT1A receptor agonists block expression of fear-potentiated startle in mice. Neuropsychopharmacology. 2003;28:654–663. doi: 10.1038/sj.npp.1300079. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe G, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeffter P, Hoyer D. Centrally acting hypotensive agents with affinity for 5-HT1A binding sites inhibit forskolin-sitimulated adenylate cyclase activity in calf hippocampus. Br J Pharmacol. 1988;95:975–985. doi: 10.1111/j.1476-5381.1988.tb11728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Bramwell SR, Grahame-Smith DG. 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br J Pharmacol. 1989;96:283–290. doi: 10.1111/j.1476-5381.1989.tb11815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibug RM, Compaan JC, Meijer OC, Van der Gugten J, Olivier B, de Kloet ER. Effects of flesinoxan on corticosteroid receptor expression in the rat hippocampus. Eur J Pharmacol. 2000;2000:111–119. doi: 10.1016/s0014-2999(00)00605-1. [DOI] [PubMed] [Google Scholar]
- Sporton SCE, Shepheard SL, Jordan D, Ramage AG. Microinjections of 5-HT1A agonists into the dorsal motor vagal nucleus produce a bradycardia in the atenolol-pretreated anaesthetized rat. Br J Pharmacol. 1991;104:466–470. doi: 10.1111/j.1476-5381.1991.tb12452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O, Misane I, Spiess J, Ogren SO. Involvement of 5-HT1A receptors in classical fear conditioning in C57BL/6J mice. J Neurosci. 2000;20:8515–8527. doi: 10.1523/JNEUROSCI.20-22-08515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong P, Greenwood B, Fleshner M. The effects of the selective 5-HT2C receptor antagonist SB 242084 on learned helplessness in male Fischer 344 rats. Psychopharmacology (Berl) 2009;203:665–675. doi: 10.1007/s00213-008-1413-3. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhou M, Liu G, Tsien J. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: A [(11)C]WAY-100635 PET investigation in healthly volunteers. Am J Psychiatry. 2001;158:1326–1328. doi: 10.1176/appi.ajp.158.8.1326. [DOI] [PubMed] [Google Scholar]
- Traber J, Glaser T. 5-HT1a receptor-related anxiolytics. Trends Pharmacol Sci. 1987;8:432–437. [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- Vieweg WV, Julius DA, Fernandez A, Beatty-Brooks M, Hettema JM, Pandurangi AK. Posttraumatic stress disorder: clinical features, pathophysiology, and treatment. Am J Med. 2006;119:383–390. doi: 10.1016/j.amjmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Youn J, Misane I, Eriksson TM, Millan MJ, Ogren SO, Verhage M, Stiedl O. Bidirectional modulation of classical fear conditioning in mice by 5-HT1A receptor ligands with contrasting intrinsic activities. Neuropharmacology. 2009;57:567–576. doi: 10.1016/j.neuropharm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Jr, Viana MB, Graeff FG. Anxiolytic effect of intra-amygdala injection of midazolam and 8-hydroxy-2-(di-n-propylamino)tetralin in the elevated T-maze. Eur J Pharmacol. 1999;369:267–270. doi: 10.1016/s0014-2999(99)00075-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang X-Y, Ye M-L, Luo C-X, Wu H-Y, Hu Y, Zhou Q-G, Wu D-L, Zhu L-J, Zhu D-Y. Neuronal nitric oxide synthase alteration accounts for the role of 5-HT1A receptor in modulating anxiety-related behaviors. J Neurosci. 2010;30:2433–2441. doi: 10.1523/JNEUROSCI.5880-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]