Abstract
House mice (genus Mus) harbor extensive allelic variation at two tandemly duplicated genes that encode the β-chain subunits of adult hemoglobin (Hb). Alternative haplotypes differ in the level of sequence divergence between the two β-globin gene duplicates: the Hbbdand Hbbphaplotypes harbor two structurally distinct β-globin genes, whereas the Hbbshaplotype harbors two β-globin duplicates that are identical in sequence. One especially salient difference between the s-type Hbs relative to the d- and p-type Hbs relates to the number of reactive β-chain cysteine residues. In addition to the highly conserved cysteine residue at β93, the d-and p-type Hbs contain an additional reactive cysteine residue at β13. To assess the functional consequences of allelic variation in β-globin cysteine content, we measured O2-binding properties and H2O2-induced oxidation rates of mono-and dicysteinyl β-Hbs from 4 different inbred strains of mice: C57BL/6J, BALB/cJ, MSM/Ms, and CAROLI/EiJ. The experiments revealed that purified Hbs from the various mouse strains did not exhibit substantial variation in O2-binding properties, but s-type Hb (which contains a single reactive β-chain cysteine residue) was far more readily oxidized to Fe3+ metHb by H2O2 than other mouse Hbs that contain two reactive β-chain cysteine residues. These results suggest that the possession of an additional reactive cysteine residue may protect against metHb formation under oxidizing conditions. The allelic differences in β-globin cysteine content could affect aspects of redox signaling and oxidative/nitrosative stress responses that are mediated by Hb-S-nitrosylation and Hb-S-glutathionylation pathways.
Keywords: Globins, hemoglobin, house mice, met-hemoglobin, Mus, oxidative stress
1. Introduction
Electrophoretic surveys of wild house mice (genus Mus) have revealed striking patterns of allelic polymorphism at two tandemly duplicated genes that encode the β-chain subunits of adult hemoglobin (Hb) (Selander and Yang, 1969; Selander et al., 1969a,b; Miyashita et al., 1985; Kawashima et al., 1995). In natural populations of Eurasian house mice, three main classes of β-globin haplotype have been characterized: Hbbd, Hbbp, and Hbbs (Erhart et al., 1985; Storz et al., 2007; Runck et al., 2009, 2010). These alternative haplotypes differ in the level of sequence divergence between the two β-globin gene duplicates, HBB-T1 and HBB-T2. The Hbbd and Hbbp haplotypes harbor two structurally distinct β-globin genes, and in each case, the more highly expressed HBB-T1 gene encodes the β-chain subunits of the major Hb isoform (isoHb) whereas HBB-T2 encodes the β-chains of the minor isoHb (Whitney, 1977; Leder et al., 1980). In contrast to the two distinct β-globin duplicates on the Hbbd and Hbbp haplotypes, the Hbbs haplotype harbors two β-globin duplicates that are identical in sequence (Erhart et al., 1985; Storz et al., 2007; Hoffmann et al. 2008). Thus, mice that are homozygous for the Hbbs haplotype synthesize a single β-chain isoHb during postnatal life.
One especially noteworthy difference between the s-type Hbs relative to the d- and p-type Hbs relates to the number of reactive β-chain cysteine residues. All of the mouse Hbs contain the highly conserved cysteine at β93(F9), which is present in the β-globins of all mammals and birds examined to date (Riggs, 1960; Reischl et al., 2007; Jensen, 2009), but the d- and p-type Hbs contain an additional reactive cysteine residue at β13(A10). The sulfhydryl –SH group of β93Cys plays an important role in the formation of mixed disulfides with low molecular mass thiols like glutathione (Murakami and Mawatari, 2003; Thomas et al., 2003; Giustarini et al., 2006; Hempe et al., 2007; Colombo et al., 2010) and the role of β93 S-nitrosylation in transducing hypoxic nitric oxide (NO) vasoactivity is a matter of ongoing debate (reviewed by Gladwin and Kim-Shapiro, 2008; Jensen, 2009; Reeder, 2010). Studies of mouse Hbs by Hempe et al. (2007) demonstrated that both β13Cys and β93Cys form mixed disulfides with glutathione under oxidizing conditions and that β13Cys is especially reactive. Thus, mice that express the d- and p-type Hbs have red cells with an elevated concentration of reactive sulfydryl groups relative to mice that only express s-type Hbs, and this results in pronounced differences in the blood-mediated metabolism of oxidants and thiol reactants such as NO and glutathione (Miranda, 2000; Giustarini et al., 2006; Hempe et al., 2007).
Results of previous studies indicated that the various Hb types of house mice exhibit slight differences in O2 affinity (Newton and Peters, 1983; Uchida et al., 1998; Runck et al., 2010). However, much more dramatic functional differences between monocysteinyl (s-type) β-Hbs and dicysteinyl (d- and p-type) β-Hbs are manifest under conditions of oxidative stress. Specifically, red cells of mice expressing dicysteinyl β-Hbs are far more susceptible to oxidant-induced hemolysis than are those of mice expressing monocysteinyl β-Hbs (Kruckeberg et al., 1987). Studies by Kruckeberg et al. (1987) demonstrated that susceptibility to red cell hemolysis was positively associated with the rate of membrane lipid oxidation, as measured by the formation of malonyldialdehyde, a fatty acid oxidative breakdown product. Red cells that were susceptible to oxidant-induced hemolysis showed a rapid rate of malonyldialdehyde formation, whereas red cells that were resistant to hemolysis showed a far lower rate of malonyldialdehyde formation. These results suggest that differences in β-globin cysteine content may be responsible for differences in heme oxidation rates, which in turn influences cell survival under oxidative stress. This is because Hbs in the ferrous Hb-Fe2+ or ferric Hb-Fe3+ (metHb) states react with hydrogen peroxide (H2O2) and/or lipid hydroperoxides to form a ferryl Fe4+ heme and a protein-centered radical in the reaction with metHb (Alayash et al., 2001). Such products can promote lipid oxidation (Harel and Kanner, 1986; Everse and Hsia, 1997; Umbreit, 2007), a process that may be further enhanced by the release of hemin from metHb subunits (Everse and Hsia, 1997; Reeder, 2010). However, experimental studies of human red cells have revealed a complex association between rates of Hb oxidation and the susceptibility to oxidant-induced hemolysis (Trotta et al., 1981, 1982, 1983). Paradoxically, red cells that accumulated a higher concentration of metHb experienced lower levels of membrane lipid oxidation. Under conditions of oxidative stress, metHb appears to protect against oxidative damage to the red cell membrane by scavenging reactive intermediates that propagate lipid peroxidation chain reactions. The oxidation of β93Cys to cysteic acid has been proposed to play a role in the scavenging of free radicals generated by reactions of ferrous and ferric heme with H2O2, thereby protecting other cellular components from oxidative damage (Jia et al., 2007; Reeder, 2010; Widmer et al., 2010). To assess the functional consequences of allelic variation in β-globin cysteine content, we measured O2-binding properties and H2O2-induced oxidation rates of mono- and dicysteinyl β-Hbs from 4 different inbred strains of mice: C57BL/6J, BALB/cJ, MSM/Ms, and CAROLI/EiJ. The experiments revealed that purified Hbs from the various mouse strains did not exhibit substantial variation in O2-binding properties, but s-type Hb (which contains a single reactive β-chain cysteine residue) was far more readily oxidized to Fe3+ by H2O2 than other mouse Hbs that contain two reactive β-chain cysteine residues. These results suggest that the possession of an additional reactive cysteine residue may protect against metHb formation under oxidizing conditions. If so, then these allelic differences in Hb oxidation rates may underlie previously documented variation in S-nitrosylation, S-glutathionylation, and the susceptibility to red cell hemolysis under oxidative stress (Kruckeberg et al., 1987; Giustarini et al., 2006; Hempe et al., 2007).
2. Materials and methods
2.1 Samples
We measured O2-binding properties and oxidation rates of purified hemolysates from 4 different inbred strains of mice: C57BL/6J, BALB/cJ, MSM/Ms, and CAROLI/EiJ. The C57BL/6J and BALB/cJ strains are referable to Mus musculus domesticus, the MSM/Ms strain is referable to M. musculus molossinus, and the CAROLI/EiJ strain is referable to M. caroli. Blood samples from each of the 4 strains were obtained from the Jackson Lab (Bar Harbor, ME, USA). Blood samples from the MSM/Ms strain were procured under a material transfer agreement with the National Institute of Genetics (Mishima, Japan).
2.2 Measurement of O2-equilibrium curves
Hemolysates were prepared according to standard methods and were stripped of organic phosphates and other ionic cofactors by passing the samples through a mixed bed resin column (MB-1 AG501-X8; BioRad, Hercules, CA, USA). The Hb samples were concentrated by ultrafiltration (cutoff 10,000), dialyzed in CO-equilibrated 10 mM HEPES buffer, pH 7.6, and stored at −80 °C as CO-derivatives. The isoHb composition of hemolysates from each mouse strain was confirmed by using thin-layer isoelectric focusing (PhastSystem, GE Healthcare Biosciences, Piscataway, NJ, USA). Using a modified diffusion chamber, O2-equilibria of Hb solutions were measured in 10 mM HEPES buffer, pH 7.4, at constant temperature, 37 °C. The met-Hb enzymatic reducing system of Hayashi et al. (1973) was used to maintain Hb in the ferrous state. Changes in the absorbance of Hb solutions were recorded in conjunction with stepwise changes in the partial pressure of O2 [PO2] of gas mixtures (prepared using cascaded Wösthoff gas-mixing pumps that perfuse the chamber; Weber, 1981, 1992; Weber et al., 2004). Values of P50 and n50 (PO2 and Hill’s cooperativity coefficient, respectively, at 50% oxygenation of the heme groups) were interpolated from linear Hill plots (log ([OxyHb]/[Hb]) vs. log PO2) based on three to five equilibration steps between 20 and 80% oxygen saturation values.
The P50 values for the stripped (i.e. cofactor-free) hemolysates provide an inverse measure of the intrinsic O2-binding affinities of the major and minor isoHbs occurring in their natural relative concentrations. To assess variation among the different mouse Hbs in the sensitivity to allosteric cofactors, we measured O2-equilibrium curves for each sample in the absence of added cofactors (stripped hemolysates), in the presence of 2,3-diphosphoglycerate (DPG; 2,3-bisphosphoglycerate), in the presence of Cl− ions (added as KCl), and in the presence of both cofactors ([Cl−], 0.10 M; [NaHEPES], 0.1 M; DPG/Hb tetramer ratio, 2.0; [Heme], 0.16 mM).
2.3 Measurement of oxidation rates in the presence of H2O2
On the day of the experiment, carboxyHb samples were converted to the oxy form in ~1 h by photodissociation on ice under air or pure O2. Full conversion to the oxy derivative was checked by UV-visible absorbance spectroscopy. Heme oxidation kinetics of oxyHb by H2O2 were measured at 37 °C by adding H2O2 (20 μM final concentration) to a solution of Hb (10 μM heme) in 0.1 M Hepes, pH 7.4. Absorbance was measured every 10 s in the range 350–500 nm using a HP 8453 diode array spectrophotometer. Kinetic traces at 430 nm were used to calculate initial rates (s−1). Statistical differences between rates (values expressed as mean ± s.e.m., with n as the number of replicates) were assessed by one-way ANOVA, using a significance threshold of α = 0.05.
2.4 Analysis of reaction products by SDS-PAGE
To assess whether the reaction between oxyHb and H2O2 promoted disfulfide polymerization, we incubated Hb (200 μM heme) in 0.1 M Hepes buffer pH 7.4, 0.1 M KCl, 100 μM DPG (DPG:Hb4 2:1) with H2O2 (400 μM) at 37 °C for 1 h and overnight. Aliquots were then mixed with SDS sample buffer containing 5 mM NEM to block free thiols, in the presence and absence of 1 mM DTT, and incubated at 100 °C for 5 min before loading on a precast PhastSystem (GE Healthcare) 10–15% SDS-PAGE for analysis of intermolecular disulfide bonds induced by reaction with H2O2.
3. Results
3.1 Patterns of β-globin sequence variation
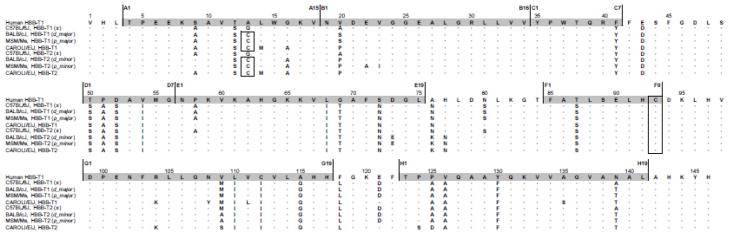
In contrast to the C57BL/6J strain, which carries a pair of tandemly duplicated β-globin genes that are identical in sequence, the BALB/cJ, MSM/Ms, and CAROLI/EiJ strains each possess structurally distinct HBB-T1 and HBB-T2 genes (Fig. 1). On the Hbbd haplotype of BALB/cJ, the HBB-T1 and HBB-T2 genes (which encode the β-chain subunits of the major and minor isoHbs [dmajor and dminor], respectively) are distinguished from one another by nine amino acid substitutions. Similar to the case with the Hbbd haplotype, the HBB-T1 and HBB-T2 genes on the Hbbp haplotype of MSM/Ms encode the β-chain subunits of the major and minor isoHbs (pmajor and pminor), respectively, and are distinguished from one another by ten amino acid substitutions. The Hbbd and Hbbp haplotypes share identical HBB-T1 sequences (dmajor = pmajor), but they are distinguished by two amino acid substitutions at HBB-T2: β22(B4)Glu→Ala and β23(B5)Val→Ile (dminor→pminor, in both cases). Thus, any functional difference between the β-chain isoHbs produced by the Hbbd and Hbbp haplotypes would have to be attributable to the two amino acid differences that distinguish the minor isoHbs, dminor and pminor. The HBB-T1 and HBB-T2 genes of CAROLI/EiJ are distinguished from one another by eight amino acid substitutions. Whereas the single Hb of C57BL/6J contains a single reactive cysteine at β93, the major and minor β-chain isoHbs expressed by the other three strains contain an additional fast-reacting cysteine residue at β13. The s-type β-globin sequence of C57BL/6J is distinguished from the HBB-T1 and HBB-T2 sequences of the other three strains by eight to eleven amino acid differences, including the presence of Gly instead of Cys at β13 (Fig. 1, Table 1). Aside from the β13Gly/Cys change, the only other consistent difference between the s-type Hb of C57BL/6J and the major Hbs of the other three strains involves the replacement of Ala for Thr at β139(H17) (Fig. 1).
Figure 1.
Structural alignment of house mouse β-globin sequences. Reactive cysteine residues at sites 13 and 93 are shown in boxes.
Table 1.
Amino acid differences between orthologs of HBB-T1 and HBB-T2 in pairwise comparisons between different stains of house mice. See Figure 1 for information about the nature of the substitutions.
| HBB-T1
| |||
|---|---|---|---|
| BALB/cJ | MSM/Ms | CAROLI/EiJ | |
| C57BL/6J | 3 | 3 | 12 |
| BALB/cJ | 0 | 10 | |
| MSM/Ms | 10 | ||
|
| |||
| HBB-T2
| |||
| BALB/cJ | MSM/Ms | CAROLI/EiJ | |
|
| |||
| C57BL/6J | 11 | 13 | 14 |
| BALB/cJ | 2 | 7 | |
| MSM/Ms | 9 | ||
3.2 Hb-O2 affinity
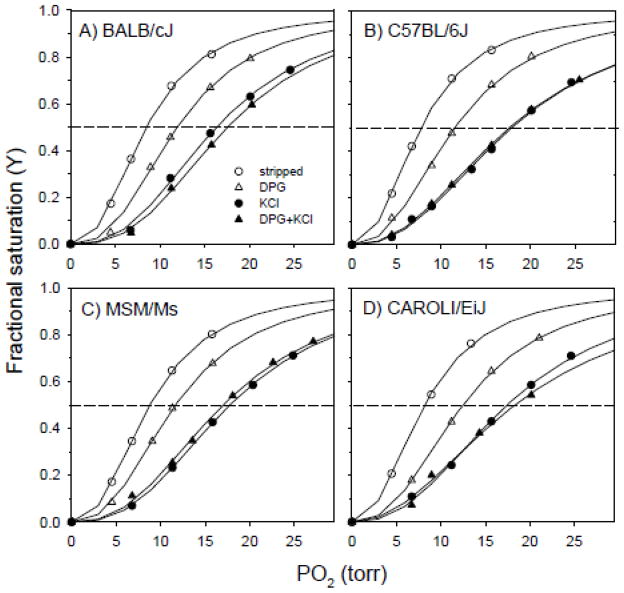
O2 equilibrium curves revealed that the purified hemolysates of the various mouse strains have fairly similar O2-binding properties. Relative to the Hbs of BALB/cJ, MSM/Ms, and CAROLI/EiJ, the s-type Hb of C57BL/6J was characterized by a slightly higher O2-affinity (lower P50) in the presence and in the absence of DPG and DPG + Cl− ions (Fig. 2, Table 2). In each strain, O2 equilibrium measurements revealed that Cl− ions exert a more potent allosteric effect than DPG. Hb-O2 affinity was always reduced to a greater extent in the presence of 0.1 mol l−1 Cl− ions than in the presence of DPG at twofold molar excess over tetrameric Hb. This is indicated by the fact that Δlog P50 (KCl-stripped) values were roughly two-fold higher than the corresponding Δlog P50 (DPG-stripped) values (Table 2). In each strain, Hb-O2 affinity was reduced to a similar extent by KCl alone and by KCl in combination with DPG (Δlog P50 [KCl-stripped] values were highly similar to Δlog P50 ([DPG+KCl]-stripped) values; Table 2). Thus, there does not appear to be any significant competition for binding sites between the monovalent and polyvalent anions.
Figure 2.
O2-equilibrium curves for stripped Hbs from four inbred strains of house mice: (A) BALB/cJ, (B) C57BL/6J, (C) MSM/Ms, and (D) CAROLI/EiJ. O2-equilibria were measured at 37° C, in 0.10 M NaHEPES buffer, at pH 7.40, in the presence and absence of allosteric cofactors [(Cl-), 0.1 M; DPG/Hb tetramer ratio, 2.0], at 0.16 mM heme concentration, and using the met-Hb reductase system of Hayashi et al. (1973).
Table 2.
O2 affinity (P50, torr; mean ±s.e.m.) and cooperativity coefficients (n50) for purified hemolysates measured in 0.1 M Hepes buffer at pH 7.40, 37 °C, in the presence of the met-Hb reducing system (Hayashi et al., 1973). Measurements were conducted in the absence of DPG and KCl (stripped), and in the presence of DPG (DPG/Hb tetramer ratio = 2.0) or 0.1 M KCl, or both effectors. [Heme], 0.16 mM. N=3 replicate measurements for C57BL/6J, BALB/cJ, and MSM/Ms.
| C57BL/6J | BALB/cJ | MSM/Ms | CAROLI/EiJ | |
|---|---|---|---|---|
| P50 (torr) | ||||
| stripped | 7.63±0.09 | 8.44±0.09 | 8.76±0.04 | 8.10 |
| DPG | 10.95±0.42 | 11.88±0.17 | 11.64±0.07 | 12.48 |
| KCl | 17.15±0.49 | 16.27±0.30 | 17.74±0.06 | 17.46 |
| DPG+KCl | 16.87±0.48 | 17.53±0.29 | 17.03±0.27 | 18.45 |
|
| ||||
| Δ log P50 | ||||
| DPG-stripped | 0.16 | 0.15 | 0.12 | 0.19 |
| KCl -stripped | 0.35 | 0.29 | 0.31 | 0.33 |
| (DPG+KCl) - stripped | 0.34 | 0.31 | 0.29 | 0.36 |
| (DPG+KCl) - KCl | −0.01 | 0.03 | −0.02 | 0.02 |
| (DPG+KCl) - DPG | 0.19 | 0.17 | 0.17 | 0.17 |
|
| ||||
| n50 | ||||
| stripped | 2.24±0.03 | 2.46±0.05 | 2.40±0.02 | 2.28 |
| DPG | 2.44±0.084 | 2.65±0.09 | 2.50±0.04 | 2.53 |
| KCl | 2.36±0.166 | 2.68±0.12 | 2.67±0.02 | 2.57 |
| DPG + KCl | 2.45±0.011 | 2.78±0.13 | 2.56±0.09 | 1.91 |
3.3 Oxidation rates
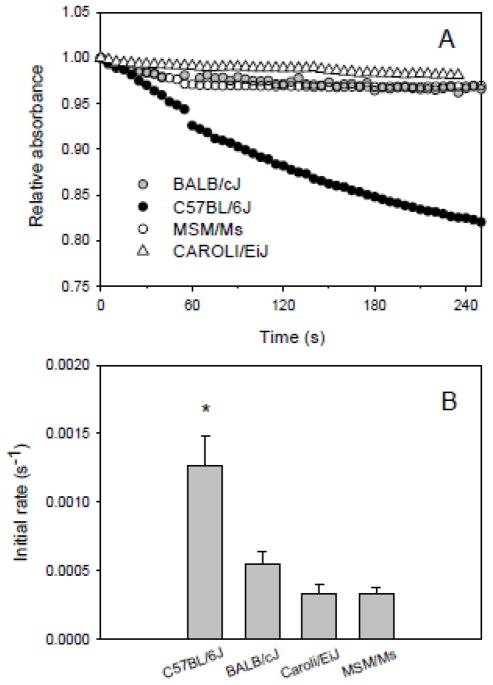
Kinetic measurements revealed that the monocysteinyl β-Hb from the strain C57BL/6J is more readily oxidized to ferric heme by H2O2 than the dicysteinyl β-Hbs from the strains BALB/cJ, MSM/Ms, and CAROLI/EiJ (Fig. 3A,B). These experiments revealed no significant variation in oxidation rates among the dicysteinyl β-Hbs from BALB/cJ, MSM/Ms, and CAROLI/EiJ. To assess whether the oxidation of cysteine –SH groups during incubation with H2O2 promoted the formation of intermolecular disulphide bonds, we analyzed reaction products by SDS-PAGE. The absence of any high-molecular weight band (~30,000; data not shown), indicated that covalent intermolecular polymerization of Hb polypeptide chains did not occur under the experimental conditions that we used.
Figure 3.
Oxidation of purified Hbs from mouse strains BALB/cJ, C57BL/6J, MSM/Ms, and CAROLI/EiJ. (A) Representative kinetic traces at 430 nm and (B) derived initial rates of oxidation (s−1) of oxyHb (10 μM heme) to metHb by H2O2 at a 1:2 molar ratio of heme:H2O2 at 37 °C in 0.1 M Hepes, pH 7.4. Values are expressed as mean ± s.e.m. Statistically significant differences (P<0.05) are denoted by an asterisk. Number of replicates (n): BALB/cJ (5), C57BL/6J (4), MSM/Ms (4), and CAROLI/EiJ (5).
4. Discussion
Results of our experiments revealed that Hbs from the various mouse strains are similar with respect to O2-binding properties, but s-type Hb from C57BL/6J (which contains a single reactive β-chain cysteine) was far more readily oxidized to Fe3+ by H2O2 than the Hbs from BALB/cJ, MSM/Ms, and CAROLI/EiJ (which contain two reactive β-chain cysteines). These results indicate that the possession of two additional reactive cysteine residues per Hb tetramer may protect against metHb formation under oxidizing conditions.
4.1 Variation in Hb-O2 affinity
O2-equilibrium measurements of the purified hemolysates revealed that the s-type Hb of C57BL/6J was characterized by a slightly higher O2-affinity than the d- and p-type Hbs of BALB/cJ, MSM/Ms, and CAROLI/EiJ (Fig. 2, Table 2). Our results are consistent with previous studies of house mouse Hbs (Uchida et al., 1998), which also showed (under different buffer conditions) a relatively weak effect of DPG on Hb-O2 affinity. Relative to Hbs of the deer mouse, Peromyscus maniculatus, a similarly sized myomorph rodent, the house mouse Hbs were characterized by similar O2 affinities in the absence of added effectors (stripped) (range of P50 [37°, pH 7.4] values = 7.63 – 8.76 for house mice and 6.70 – 8.56 for deer mice). However, house mouse Hbs are characterized by substantially lower O2 affinities in the presence of DPG and Cl− ions (range of P50 [37°, pH 7.4] values = 16.87 – 18.45 for house mice and 11.71 – 15.76 for deer mice; Storz et al., 2009, 2010). One unusual property that is shared between the Hbs of deer mice and house mice is that in both cases Cl− ions exert a stronger allosteric effect than DPG. This is an important finding because DPG is a much more potent allosteric effector than Cl− ions in the overwhelming majority of mammalian Hbs. The only documented exceptions include Hbs from select lineages of artiodactyls, carnivores, moles, and prosimian primates (Bunn, 1971; Taketa et al., 1971; Bunn et al., 1974; Campbell et al., 2010).
4.2 Variation in oxidation rates
Results of our experiments revealed far higher rates of H2O2-induced heme oxidation in mouse strains with monocysteinyl β-globins (C57BL/6J) than in mouse strains with dicysteinyl β-globins (BALB/cJ, MSM/Ms, and CAROLI/EiJ). This dichotomy in the rate of heme oxidation is mirrored by previously documented variation among mouse strains in red cell survival under oxidative stress: strains like C57BL/6J with monocysteinyl β-globins proved highly resistant to oxidant-induced hemolysis, whereas strains like BALB/cJ with dicysteinyl β-globins proved far more susceptible to hemolysis (Kruckeberg et al., 1987). Our results, combined with the experimental results of Kruckeberg et al. (1987), suggest that the elevated oxidation rate of the monocysteinyl β-chain Hb is associated with increased resistance to hemolysis under conditions of oxidative stress. This is consistent with the hypothesis of Trotta et al. (1981, 1982, 1983) that, under conditions of oxidative stress, an increased concentration of metHb may confer a protective effect at the cellular level by inhibiting lipid peroxidation.
4.3 Structural mechanisms of thiol reactivity and heme oxidation
In mouse Hb, the especially fast reactivity of β13Cys relative to β93Cys appears to be attributable to an increased solvent accessibility and an increased electron density around the sulfur atom of the β13Cys thiol group due to the formation of a hydrogen bond with the carbonyl of β10(A7)Ala (Miranda, 2000). The β13Gly→Cys residue change likely confers protection against H2O2-induced heme oxidation because H2O2 reacts with the thiol instead of the heme. Since Hb is primarily oxidized by H2O2 in the deoxy-ferrous state, the initial oxidation of Fe2+ to Fe3+ should be inversely proportional to O2 affinity (Alayash et al., 1999). However, our experiments revealed that the Hb type with the highest oxidation rate was also characterized by the highest O2 affinity (lowest P50) at physiological pH (Table 2), so the observed differences in oxidation rates cannot be explained by differences in Hb-O2 affinity.
4.4 Possible adaptive significance of the allelic variation in oxidation rates
Electrophoretic surveys of β-globin polymorphism in natural populations of Mus musculus and M. domesticus have revealed that the Hbbd and Hbbs haplotypes are consistently present at intermediate frequencies in population samples from across the species’ range, a pattern that is not mirrored by other unlinked autosomal genes (reviewed by Storz et al. 2007). This striking uniformity of allele frequencies has led a number of authors to conclude that the two-locus β-globin polymorphism may be maintained by some form of balancing selection (Berry, 1978). This hypothesis is supported by analyses of nucleotide variation at the HBB-T1 and HBB-T2 genes, which have revealed remarkably high levels of nucleotide diversity within species (Storz et al., 2007) and the pervasive sharing of Hbbd and Hbbs haplotypes among multiple Eurasian species in the subgenus Mus (Runck et al., 2009). However, despite the abundance of indirect evidence for the selective maintenance of the Hbbd and Hbbs haplotypes, there is currently no widely accepted mechanistic explanation for the possible existence of fitness variation among mice with different β-globin genotypes. One possibility is that the coding mutations in the HBB genes do not directly contribute to fitness variation, but are selectively maintained due to close physical linkage with other (possibly noncoding) sites that represent the true target of balancing selection (Runck et al., 2010). This seems especially plausible since the alternative s- and d-type alleles at the two tandemly linked HBB genes are associated with differences in Hb concentration (Peters et al., 2010). Our results and those of other recent studies (Giustarini et al., 2006; Hempe et al., 2007) suggest that the adaptive significance of the two-locus β-globin polymorphism could relate specifically to allelic differences in oxidation rate (mediated by β-globin cysteine content), and may therefore revolve around a signaling function of the Hb-metHb redox couple. Since the intraerythrocytic concentration of reactive sulfydryl groups influences the availability of reduced glutathione for enzymatic detoxification reactions (Di Simplicio et al., 1998; Murakami and Mawatari, 2003; Dalle-Donne et al., 2007; Giustarini et al., 2006; Hempe et al., 2007; Colombo et al., 2010), allelic variation in β-globin cysteine content may contribute to variation in the cellular response to pathogenic infection and oxidative/nitrosative stress. The differences in met-Hb formation that we documented between strains of mice that express mono- and dicysteinyl β-chain Hbs suggest that the extensive variation in Hb cysteine content among different vertebrate taxa (Reischl et al. 2007) may be associated with equally extensive variation in the redox activity of red blood cells.
Acknowledgments
We thank three anonymous reviewers for helpful comments and suggestions, and we thank Mai-Britt Hemmingsen for valuable assistance in the lab. This work was funded by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (R01 HL087216 and HL087216-S1) and the National Science Foundation (IOS-0949931).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alayash AI, Patel RP, Cashon RE. Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid Redox Signal. 2001;3:313–327. doi: 10.1089/152308601300185250. [DOI] [PubMed] [Google Scholar]
- Alayash AI, Ryan BAB, Eich RF, Olson JS, Cashon RE. Reactions of sperm whale myoglobin with hydrogen peroxide - Effects of distal pocket mutations on the formation and stability of the ferryl intermediate. J Biol Chem. 1999;274:2029–2037. doi: 10.1074/jbc.274.4.2029. [DOI] [PubMed] [Google Scholar]
- Berry RJ. Genetic variation in wild house mice: where natural selection and history meet. Am Sci. 1978;66:52–60. [PubMed] [Google Scholar]
- Bunn HF. Differences in the interaction of 2,3-diphosphoglycerate with certain mammalian haemoglobins. Science. 1971;172:1049–1052. doi: 10.1126/science.172.3987.1049. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Seal US, Scott AF. Role of 2,3-diphosphoglycerate in mediating hemoglobin function of mammalian red cells. Ann N Y Acad Sci. 1974;241:498–512. doi: 10.1111/j.1749-6632.1974.tb21906.x. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Storz JF, Signore AV, Moriyama H, Catania KC, Payson AP, Bonaventura J, Stetefeld J, Weber RE. Molecular basis of a novel adaptation to hypoxic-hypercapnia in a strictly fossorial mole. BMC Evol Biol. 2010;10:14. doi: 10.1186/1471-2148-10-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Dalle-Donne I, Giustarini D, Gagliano N, Portinaro N, Colombo R, Rossi R, Milzani A. Cellular redox potential and hemoglobin S-glutathionylation in human and rat erythrocytes: A comparative study. Blood Cells Mol Dis. 2010;44:133–139. doi: 10.1016/j.bcmd.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-Glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Di Simplicio P, Cacace MG, Lusini L, Giannerini F, Giustarini D, Rossi R. Role of protein -SH groups in redox homeostasis - the erythrocyte as a model system. Arch Biochem Biophys. 1998;355:145–152. doi: 10.1006/abbi.1998.0694. [DOI] [PubMed] [Google Scholar]
- Erhart MA, Simons KS, Weaver S. Evolution of mouse β-globin genes: a recent gene conversion in the Hbbs haplotype. Mol Biol Evol. 1985;2:304–320. doi: 10.1093/oxfordjournals.molbev.a040353. [DOI] [PubMed] [Google Scholar]
- Everse J, Hsia N. The toxicities of native and modified hemoglobins. Free Radic Biol Med. 1997;22:1075–1099. doi: 10.1016/s0891-5849(96)00499-6. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Dalle-Donne I, Cavarra E, Fineschi S, Lungarella G, Milzani A, Rossi R. Metabolism of oxidants by blood from different mouse strains. Biochem Pharmacol. 2006;71:1753–1764. doi: 10.1016/j.bcp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel S, Kanner J. Hydrogen peroxide-activated methaemoglobin and other methaemoproteins as initiators of membranal lipid peroxidation. In: Rotilio G, editor. Superoxide and Superoxide Dismutase in Chemistry, Biology, and Medicine. New York: Elsevier Science; 1986. pp. 25–28. [Google Scholar]
- Hayashi A, Suzuki T, Shin M. An enzymatic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim Biophys Acta. 1973;310:309–316. doi: 10.1016/0005-2795(73)90110-4. [DOI] [PubMed] [Google Scholar]
- Hempe JM, Ory-Ascani J, Hsia D. Genetic variation in mouse beta globin cysteine content modifies glutathione metabolism: Implications for the use of mouse models. Exp Biol Med. 2007;232:437–444. [PubMed] [Google Scholar]
- Hoffmann FG, Opazo JC, Storz JF. New genes originated via multiple recombinational pathways in the β-globin gene family of rodents. Mol Biol Evol. 2008;25:2589–2600. doi: 10.1093/molbev/msn200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J Exp Biol. 2009;212:3387–3393. doi: 10.1242/jeb.023697. [DOI] [PubMed] [Google Scholar]
- Jia YP, Buehler PW, Boykins RA, Venable RM, Alayash AI. Structural basis of peroxide-mediated changes in human hemoglobin - A novel oxidative pathway. J Biol Chem. 2007;282:4894–4907. doi: 10.1074/jbc.M609955200. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Miyashita N, Tsuchiya K, Li H, Wang G, Wang CH, Wu X-L, Wang C, Jin M-L, He X-Q, et al. Geographical distribution of the Hbb haplotypes in the Mus musculus subspecies in Eastern Asia. Jpn J Genet. 1995;70:17–23. doi: 10.1266/jjg.70.17. [DOI] [PubMed] [Google Scholar]
- Kruckeberg WC, Doorenbos DI, Brown PO. Genetic differences in hemoglobin influence on erythrocyte oxidative stress hemolysis. Blood. 1987;70:909–914. [PubMed] [Google Scholar]
- Leder P, Hansen JN, Konkel D, Leder A, Nishioka Y, Talkington C. Mouse globin system: a functional and evolutionary analysis. Science. 1980;209:1336–1342. doi: 10.1126/science.7414319. [DOI] [PubMed] [Google Scholar]
- Miranda JJ. Highly reactive cysteine residues in rodent hemoglobins. Bioch Biophys Res Comm. 2000;275:517–523. doi: 10.1006/bbrc.2000.3326. [DOI] [PubMed] [Google Scholar]
- Miyashita N, Moriwaki K, Minezawa M, Yonekawa H, Bonhomme F, Migita S, Yu Z, Lu D, Wang SC, Thohari M. Allelic constitution of the hemoglobin beta chain in wild populations of the house mouse, Mus musculus. Biochem Genet. 1985;23:975–986. doi: 10.1007/BF00499941. [DOI] [PubMed] [Google Scholar]
- Murakami K, Mawatari S. Oxidation of hemoglobin to methemoglobin in intact erythrocyte by a hydroperoxide induces formation of glutathionyl hemoglobin and binding of α-hemoglobin to membrane. Arch Biochem Biophys. 2003;417:244–250. doi: 10.1016/s0003-9861(03)00389-8. [DOI] [PubMed] [Google Scholar]
- Newton MF, Peters J. Physiological variation of mouse haemoglobin. Proc Roy Soc Lond B. 1983;218:443–453. doi: 10.1098/rspb.1983.0050. [DOI] [PubMed] [Google Scholar]
- Peters LL, Shavit JA, Lambert AJ, Tsaih SW, Li QA, Su ZG, Leduc MS, Paigen B, Churchill GA, Ginsburg D, et al. Sequence variation at multiple loci influences red cell hemoglobin concentration. Blood. 2010;116:E139–E149. doi: 10.1182/blood-2010-05-283879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder BJ. The redox activity of hemoglobins: From physiologic functions to pathologic mechanisms. Antioxid Redox Signaling. 2010;13:1087–1123. doi: 10.1089/ars.2009.2974. [DOI] [PubMed] [Google Scholar]
- Reischl E, Dafre AL, Franco JL, Wilhelm D. Distribution, adaptation and physiological meaning of thiols from vertebrate hemoglobins. Comp Biochem Physiol C. 2007;146:22–53. doi: 10.1016/j.cbpc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Riggs A. The nature and significance of the Bohr effect in mammalian hemoglobins. J Gen Physiol. 1960;43:737–752. doi: 10.1085/jgp.43.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runck AM, Moriyama H, Storz JF. Evolution of duplicated β-globin genes and the structural basis of hemoglobin isoform differentiation in Mus. Mol Biol Evol. 2009;26:2521–2532. doi: 10.1093/molbev/msp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runck AM, Weber RE, Fago A, Storz JF. Evolutionary and functional properties of a two-locus β-globin polymorphism in Indian house mice. Genetics. 2010;184:1121–1131. doi: 10.1534/genetics.109.113506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R, Yang S, Hunt W. Polymorphisms in esterases and hemoglobin in wild populations of the house mouse (Mus musculus) Univ Texas Publ. 1969a;6918:271–338. [Google Scholar]
- Selander RK, Hunt WG, Yang SY. Protein polymorphism and genic heterozygosity in two European subspecies of the house mouse. Evolution. 1969b:379–390. doi: 10.1111/j.1558-5646.1969.tb03522.x. [DOI] [PubMed] [Google Scholar]
- Selander RK, Yang SY. Protein polymorphism and genic heterozygosity in a wild population of the house mouse (Mus musculus) Genetics. 1969;63:653–667. doi: 10.1093/genetics/63.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Baze M, Waite JL, Hoffmann FG, Opazo JC, Hayes JP. Complex signatures of selection and gene conversion in the duplicated globin genes of house mice. Genetics. 2007;177:481–500. doi: 10.1534/genetics.107.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol. 2010;213:2565–2574. doi: 10.1242/jeb.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, Moriyama H, Weber RE, Fago A. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci USA. 2009;106:14450–14455. doi: 10.1073/pnas.0905224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketa F, Mauk AG, Lessard JL. β chain amino termini of the cat hemoglobins and the response to 2,3-diphosphoglycerate and adenosine triphosphate. J Biol Chem. 1971;246:4471–4476. [PubMed] [Google Scholar]
- Thomas JA, Mallis RJ, Sies H. Protein S-thiolation, S-nitrosylation, and irreversible sulfydryl oxidation: roles in redox regulation. In: Gitler C, Danon A, editors. Cellular Implications of Redox Signaling. Imperial College Press; London: 2003. pp. 141–174. [Google Scholar]
- Trotta RJ, Sullivan SG, Stern A. Lipid peroxidation and hemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide: Dependence on glucose metabolism and hemoglobin status. Biochim Biophys Acta. 1981;679:230–237. doi: 10.1016/0304-4165(81)90211-7. [DOI] [PubMed] [Google Scholar]
- Trotta RJ, Sullivan SG, Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide: Effects of the hexose monophosphate shunt as mediated by glutathione and ascorbate. Biochem J. 1982;204:405–415. doi: 10.1042/bj2040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta RJ, Sullivan SG, Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide: The relative roles of haem- and glutathione-dependent decomposition of t-butyl hydroperoxide and membrane lipid hydroperoxides in lipid peroxidation and haemolysis. Biochem J. 1983;212:759–772. doi: 10.1042/bj2120759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Reilly MP, Asakura T. Molecular stability and function of mouse hemoglobins. Zool Sci. 1998;15:703–706. [Google Scholar]
- Umbreit J. Methemoglobin - It’s not just blue: A concise review. Am J Hematol. 2007;82:134–144. doi: 10.1002/ajh.20738. [DOI] [PubMed] [Google Scholar]
- Weber RE. Cationic control of oxygen affinity in lugworm erythocruorin. Nature. 1981;292:386–387. [Google Scholar]
- Weber RE. Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J Appl Physiol. 1992;72:1611–1615. doi: 10.1152/jappl.1992.72.4.1611. [DOI] [PubMed] [Google Scholar]
- Weber RE, Voelter W, Fago A, Echner H, Campanella E, Low PS. Modulation of red cell glycolysis: interactions between vertebrate hemoglobins and cytoplasmic domains of band 3 red cell membrane proteins. Am J Physiol- Regul Integr Comp Physiol. 2004;287:R454–R464. doi: 10.1152/ajpregu.00060.2004. [DOI] [PubMed] [Google Scholar]
- Whitney JB. Differential control of the synthesis of two hemoglobin beta chains in normal mice. Cell. 1977;12:863–871. doi: 10.1016/0092-8674(77)90150-7. [DOI] [PubMed] [Google Scholar]
- Widmer CC, Pereira CP, Gehrig P, Vallelian F, Schoedon G, Buehler PW, Schaer DJ. Hemoglobin can attenuate hydrogen peroxide-induced oxidative stress by acting as an antioxidative peroxidase. Antioxid Redox Signal. 2010;12:185–198. doi: 10.1089/ars.2009.2826. [DOI] [PubMed] [Google Scholar]