Abstract
With the reason that systemically administered adenovirus (Ad) is rapidly extinguished by innate/adaptive immune responses and accumulation in liver, in vivo application of the Ad vector is strictly restricted. For achieving to develop successful Ad vector systems for cancer therapy, the chemical or physical modification of Ad vectors with polymers has been generally used as a promising strategy to overcome the obstacles. With polyethylene glycol (PEG) first in order, a variety of polymers have been developed to shield the surface of therapeutic Ad vectors and well accomplished to extend circulation time in blood and reduce liver toxicity. However, although polymer-coated Ads can successfully evacuate from a series of guarding systems in vivo and locate within tumors by enhanced permeability and retention (EPR) effect, the possibility to entering into the target cell is few and far between. To endow targeting moiety to polymer-coated Ad vectors, a diversity of ligands such as tumor-homing peptides, growth factors or antibodies, have been introduced with avoiding unwanted transduction and enhancing therapeutic efficacy. Here, we will describe and classify the characteristics of the published polymers with respect to Ad vectors. Furthermore, we will also compare the properties of variable targeting ligands, which are being utilized for addressing polymer-coated Ad vectors actively.
1. Introduction
Adenovirus (Ad) has long been predicted as an oncolytic instrument soon after it was discovered in 1953 by Wallace Rowe and his colleagues (1, 2). With the reason that adenoidal-pharyngeal-conjuctival virus (APC, now known to be an Ad) can cause cytopathogenic effect in tissue culture, the virus was rapidly used in clinical trials for the treatment of cervical cancer in 1956 (2). In 26 out of 40 patients inoculated with wild type Ad, localized necrosis was found in tumors within 10 days; more interestingly, the areas of necrosis appeared to be restricted to the cancerous tissue. Even though those who responded to Ad administered by intravenous, intravascular or intra-arterial routes showed striking effects, the survival rate of these patients was not significantly extended (2, 3). Because administered Ad was quickly eradicated by human immune systems since infants and children are most commonly affected by Ads, the continued investigations using Ad for the treatment of cervical cancer did not prolonged the survival (4, 5). However, there might be no doubt that Ad can be used for anti-cancer therapeutic agents.
Since the results of clinical gene therapy trials began to appear in 1989, the number of gene therapy clinical trials using Ad vectors worldwide has reached 414 with taking the first ranking (more than 24% of all cases including viral & non-viral vectors) (http://www.wiley.co.uk/genmed/clinical/). Practically, developments of recombinant Ad vector systems and their therapeutic applications have been mostly focused on human cancers. For just delivering genetic materials using Ad vectors, E1 region-, which encodes important proteins for cellular transformation and viral replication, deleted replication-incompetent Ad vectors have been chiefly utilized, before the concept of the oncolytic Ad emerged for cancer gene therapy (6). Although Ad vectors have many fascinating advantages such as an efficient nuclear entry mechanism, high gene transduction efficiency and the ability to concentrated at high titers, the efficacy and duration of transgene expression are very limited when replication-incompetent Ad is used (7).
By taking advantage of the dysfunctional defense mechanisms such as endogenous tumor suppression proteins (p53, pRb, p14ARF, etc.) in cancer cells, but the intact ones in normal cells, oncolytic Ad has been first introduced by Bischoff group in 1996 that E1B 55kDa-deleted adenovirus can replicates in and kills p53-deficient human tumor cells (8, 9). Soon afterwards, many kinds of oncolytic Ad vector systems have been developed by genetic modification of Ad genome (10). Briefly, the development of oncolytic Ad vector system has been progressed to the following two directions: 1) modulation of E1 genes such as deletion of E1B 55 kDa and/or 19 kDa genes, deletion or substitution of pRb-binding sites of E1A gene (8, 11, 12); 2) introduction of tumor specific promoter/enhancer derived from prostate-specific antigen (PSA) (13), α-fetoprotein (AFP) (14), carcinoembryonic antigen (CEA) (15), epithelial mucin (MUC1) (16), human telomerase reverse transcriptase (hTERT) or hypoxia responsive element (HRE) (17, 18).
Cancer-specifically replicating Ad has much more benefits to kill cancer cells when compared with non-replicating Ad, owing to the ability of cancer-selective replication of viral genome by using host-transcriptional machinery and of maximized transgene expression by multiplication of viral genome including therapeutic-transgene expression cassette (over 10,000 copies of wild type Ad genome per single cell) (19). A variety of technologies to enhance cancer-killing potency of Ad vector currently under development can be briefly classified into the following categories: (1) introduction of cytotoxic genes such as tumor-suppressor gene (p53) (20), suicide gene (herpes simplex virus-thymidine kinase) (HSV-TK) (21), cytosine deaminase (CD) (22), tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (23); (2) overexpression of immune-stimulatory cytokine genes, including interleukin-2 (IL-2), IL-12, IL-18, interferon (IFN)-α, etc. (24, 25); (3) down-regulation of sequence-specific gene expression by small interfering RNA (siRNA) against mRNA related with angiogenesis (26, 27); (4) elimination of the extracellular matrix barrier within tumor for efficient viral spread (Ad death protein (ADP), relaxin, decorin) (28-30).
Despite all efforts, almost all Ad delivered systemically is vanished immediately (Ad half-life is less than 2 min) (31). In accordance with Ad vector has many obstacles that strongly limit its efficient and safe application in vivo, which is why human clinical trials are strictly limited to local injection only against exposed tumors such as head and cancer patients (32). There are the following three reasons that administered Ad is quickly cleared biologically before the virus reaches and infects to where we want to target like tumor: (1) innate immune response by macrophages and dendritic cells (DC); (2) adaptive immune response by neutralizing anti-Ad antibody (Ab); (3) infection to unwanted organs such as liver accumulation by absence of targeting moiety. Once Ad vectors are administered intravenously, numerous antigen-presenting cells such as macrophages and DC are waiting for foreign pathogens in the first gateway, resulting in the release of proinflammatory cytokines and chemokines like IL-6, TNF-a and IFN-g inducible protein-10 (33-35). Activation of the complement system has also been reported to contribute to the induction of innate immune system (36). In the second gateway, more specific interaction for inactivating Ad is participated by widespread neutralizing anti-Ad Ab, which is exhibited in individuals who have previously been exposed to Ad (37). The major components of Ad capsid, hexon proteins have been well known that are high immunogenic components because they are recognized by most of the Abs among all anti-Ad Abs (38). In company with innate immune response, the induction of adaptive immune response has also been reported to be another important obstacle-step to reduce the Ad-delivery efficiency. Although Ad vectors evade all innate/adaptive immune system, almost all of them accumulate in liver followed by acute liver toxicity and leftover Ads locate to anywhere in the body (39). Passively accumulated Ads within solid tumor have a possibility to infect to cancer cells as a result of the poor lymphatic drainage leading to retention of macromolecules within the tumor stroma as well as the leaky vasculature giving high levels of fluid extravasation (40). This enhanced permeability and retention (EPR) effect is a well-documented phenomenon, which is exploited for Gadolinium imaging and proposed for therapeutic use by Dr. Maeda in the middle of 1980s (41). However, barely remaining Ad located in tumor cells is not sufficient to show therapeutic efficacy.
Whereas many approaches for arming Ad vectors to enhance the killing efficacy against cancer have been reported, a variety of technologies for shielding Ads to reduce host immune responses have been developed from various angles as well (42-45).In contrast with the engineering Ad-tropism at the genetic level, which has problems such as difficulty to remove immune-recognition sites, production of much increased amounts of non-infectious Ad particles versus infectious viral particles and batch-to-batch difference of the vectors (46-48), the techniques for coating Ad with synthetic polymers attenuating the ability of Ad to be inactivated by antigen-presenting cells or neutralizing Abs, have been successfully accomplished (42-44) as the beginning of the covalent chemical modification of Ad vectors with polyethylene glycol (PEG; PEGylation) (49).
In this review, we will describe the current achievements and potentials of the techniques for shielding and addressing Ad vectors with passively or actively targeted polymers used so far, which are classified with characteristics of the polymers for passive targeting or of the targeting ligands conjugated to the polymers for active targeting. For developing successful therapeutic agents using Ad vectors, both shielding with appropriate non-cytotoxic polymers (Safety) and addressing with specific targeting ligands (selective therapeutic efficacy) are inescapable to delivering Ad vectors safely and efficiently in vivo.
2. Passive targeting
Many types of viruses including Ad have been modified to eliminate their toxicity with maintaining their high gene transfer capability. The obstacles of Ad vectors for broad clinical utility have not yet been successfully achieved due to the problems related to the inherent properties of Ad such as liver-tropism and fast eradication by anti-Ad Abs. To overcome the limits of the Ad vectors for in vivo application, in terms of safety and efficacy issues caused by immunogenicity of their inherent tropisms, combinatorial approaches with non-viral vector system have been led as an alternative.
Accumulation of macromolecules containing therapeutic gene are well understood to able to locate within tumor via the EPR effect called as passive targeting, which is caused by the leaky blood vessels coupled with poor lympathic evacuation in the tumors (40, 41). Generally, two kinds of capsid modification approaches using polymers have been reported to shield Ad vectors safely and efficiently, which are based respectively on chemical or physical engineering, since genetic engineering of Ad genome to evade immune-recognition is very difficult and inefficient at overcoming these problems (50). These engineering methods to modify Ad with polymers are fully required to protect Ad vectors for evading innate and adaptive immune responses and also to target Ad vectors towards cells where it can mediate a useful outcome. In this part, we will classify and present the Ad-capsid modification engineering used for coating Ad with polymers chemically or physically, so far (Table 1).
Table 1. Classification of polymers coated with Ad vectors for passive targeting.
| Polymer type | Complex formation | Ad type | Characterizations | Applications | Ref |
|---|---|---|---|---|---|
| PEG | Chemical | Ad2-ΔE1/β-gal | MPEG, CC-MPEG, TMPEG, and SS-MPEG | in vitro and in vivo | (49) |
| Ad-ΔE1/GFP/Luc | SPA-PEG (5 k) | in vitro and in vivo | (53) | ||
| Ad-ΔE1/GFP | SPA-PEG (2, 5 or 20 k) | in vivo | (54) | ||
| Geneti-Chemical | Cystein-incorporated Ad-ΔE1/GFP | PEGylation onto thiol groups in HI loop of Ad capsid | in vitro | (60) | |
| Ad-ΔE1/Luc/BAP-L2 | PEGylation to BAP incorporated in hexon (Avidin-biotin) | in vivo | (61) | ||
| HPMA | Chemical | Ad-ΔE1/GFP, Ad-ΔE1/β-gal | reactive 4-nitrophenoxy groups on pHPMA | in vivo | (52) |
| Physi-chemical | Ad-ΔE1/Luc | amine reactive carbonyl thiazolidine-2-thione (TT) groups and/or positively charged quaternary ammonium (QA) groups | ex vivo | (64) | |
| PEI | Physical | Ad-ΔE1/ΔE4 | PEI-DNA-Ad complex | in vitro | (67) |
| Ad-ΔE1/GFP, Ad-ΔE1/SDF1a | Biodegradable PEI (2 k)-conjugated with DEG | in vivo | (162) | ||
| PLL | Physical | Ad2-ΔE1/β-gal, Ad-ΔE1/β-gal, Ad2-ΔE1/CFTR | PLL-Ad complex | in vitro and in vivo | (74) |
| Ad-ΔE1/GFP | Two types of PEG-grafted PLLs | in vitro | (77) | ||
| Lipid | Physical | Ad2-ΔE1/β-gal | GL-67/DOPE-PEG | in vivo | (78) |
| Ad-ΔE1/β-gal | DMPC-Chol-DSPE(PEG) (several lipid components) | in vivo (biodistribution) | (79) | ||
| Alginate | Encapsulation | Ad-ΔE1/β-gal | biodegradable alginate microparticles | in vivo | (98) |
| Chitosan | Physical | Ad-ΔE1/β-gal | variable sizes of natural chitosan | in vitro | (80) |
| Ad-ΔE1/GFP | folate receptor-targeted PEG-chitosan | in vitro | (81) | ||
| ABP | Physical | Ad-ΔE1/GFP | ABP-coated Ad complex | in vitro | (90) |
| chemical | Ad-ΔE1/GFP, YKL-1001 | ABP-conjugated YKL-1001 complex | in vitro and in vivo | (91) |
Ad2-ΔE1: E1-deleted replication deficient Ad serotype 2
Ad-ΔE1: E1-deleted replication deficient Ad serotype 5
β-gal: β-galactosidase gene
GFP: green fluorescent protein gene
Luc: firefly luciferase gene
CFTR: cystic fibrosis transmembrane conductance regulator gene
YKL-1001: hepatoma-specific oncolytic Ad
2.1. Chemical engineering by polymers
Chemical engineering of virus particles with hydrophilic polymers provides an opportunity to mask the viral surface epitopes, preventing Ab-neutralization and reducing unwanted interactions with blood components after systemic administration. Chemical engineering for surface modification of Ad vectors is mainly carried out by cross-linking via amine functional groups on the Ad surface (approximately 1,800 free amines on the hexon, penton and fiber proteins). In many papers, PEG and poly-N-(2-hydroxypropyl)methacrylamide (pHPMA) are described as the representative polymers of chemical engineering to Ad.
2.1.1. PEG
The first attempt to PEGylate Ad vectors were performed by O'Riordan et al. in 1999, on the basis of previous experience with the modification of therapeutic proteins in general (49). According to their results, TM-PEG was shown to be a suitable reagent for PEGylation of amino groups on the surface of viral capsids based on Ad serotype 5, while allowing for retention of biological Ad activity. Reaction to PEG has been shown to improve the in vivo pharmacokinetics of the administered vector by increasing the clearance time of the vector in the blood (31), with preventing anti-Ad antibody neutralization (51, 52) and decreasing the innate immune response against the vector (53).
Recent studies have showed how PEG size and the degree of PEGylation affect in vivo transduction of PEGylated Ad. At early, PEGylated Ad had been studied with small sized PEG (2-5 k), although PEG can be synthesized to various molecular weights and lengths of up to 40 k. Also, it can be modified branch types or PEG that contains different active groups on each side chain. Wortmann et al. reported that the size of the PEG determines the detargeting efficiency (54). In this study, small sized PEG (2 k) did not mediate significant detargeting to liver. This result is corresponded with the results published by Mok et al. who reported that small 5 k-SPA-PEG could not prevent the accumulation in the liver after intravenous injection (53). On the contrary, their results demonstrated that 20 k PEG greatly decreases liver transduction in vivo. These results are also agreed with the recent study by Hofherr et al. In their study, the effect of the different-sized PEGs (2, 20 or 35 k-PEG) was tested on in vivo liver transduction after i.v. injection (55). Stronger level of luciferase expression in liver was observed with 10-fold higher in 5 k PEGylated Ad group than those in 20 or 35 k PEG-Ad groups. Their results indicate that the larger sized PEG reduces liver transduction, resulting in the prolonged circulation time of Ad in bloodstream because of preventing the accumulation of Ad to the liver.
In general, the degree of PEGylation affects in vivo transduction of PEGylated Ad. Gao et al. and Yao et al. groups reported that the accumulation in tumor and half-life in blood of PEGylated Ad are dependent of the ratio of the PEG contents (56, 57). These observations are also associated with the size of PEGylated Ad. The increased size of PEGylated Ad indicates the expanded particle diameter (56, 58). Because the size of liver fenestrations allowing access from the blood to the liver parenchyma is 100-110 nm in mice (59), PEGylated Ad larger than this size can avoid the liver sinusoid fenestrae. So, PEGylated Ad that is smaller than < 200 nm is shown the increased tumor accumulation via EPR effect through prolonged circulation time in blood.
In almost all studies about PEGylation of Ad, PEG has been covalently conjugated with amine group of lysine residues on Ad capsid (hexon, penton, and fiber proteins). However, this masking effect by PEGylation rather inhibits the attachment of the Ad to coxsackievirus and Ad receptor (CAR) for the natural cell entry pathway, resulting in significant reduction in the transduction efficiency with increasing the PEGylation ratio. These results have demonstrated by many studies (53, 54, 56) that more improved method without decreased transduction efficiency is needed for PEGylation of Ad. In order to make conventional PEGylated Ad vectors, PEG has to be selectively conjugated and controlled to specific sites on the Ad surface besides the methods attaching retargeting ligands to the ends of PEG chains for active targeting. Kreppel et al. introduced the specific method called geneti-chemical strategy (60). They combined the chemical modification of Ad with genetically introduced cysteine residues into the HI-loop of the fiber protein. In this study, chemical reactivity of the genetically introduced thiol groups on the surface of Ad particle could be controlled without affecting the structural and biological nature of Ad, resulting in offering the high specificity for active targeting.
Recently, in order to selectively conjugate PEG to hexon protein on Ad surface using avidin-biotin interaction, Kouyama et al. generated Ad-BAP-L2 (61). This virus was genetically incorporated the biotin acceptor peptide (BAP) into the hexon hypervariable region 5 (HVR5) of the hexon, and then, PEG was specifically conjugated to the hexon HVR5 via avidin-biotin complex formation. In this study, hexon on Ad surface recognized as the target site of immune system specifically masked by PEG, resulting in lower immunogenicity and the reduction in the hepatic transduction compared with those of its control virus.
The amount of PEGylation and the molecular size of PEG including PEGylation of Ad not mentioned above should be considered to overcome the hurdles imposed by the high promiscuity of Ad vectors. These technologies may serve as tools for more development of the conventional adenoviral vectors.
2.1.2. poly-N-(2-hydroxypropyl) methacrylamide (pHPMA)
As another representative polymer for chemical engineering, pHPMA is a multivalent reactive hydrophilic polymer to be developed as a drug carrier including chemotherapeutic agents (43). pHPMA based copolymers is variously modified to shield Ad surface against preexisting neutralizing antibodies, complement, platelets or erythrocytes for systemic injection. Fisher et al. generated pHPMA-ONp as addition to reactive 4-nitrophenoxy groups on pendent diglycyl side chains of pHPMA (52). In this study, modified pHPMA carrying Ad vectors accomplished the abilities evading Ab recognition and abrogating CAR-binding followed by the Ads infection to the cells, but make it possible for retargeting by introduction of ligands against target cell surface. Also, hepatic transgene expression of pHPMA-coated Ad was examined over 100-fold lower than that of naked Ad, resulting in the induction of prolonged circulation time in blood (62). Moreover, the accumulation of Ad in the tumor after intravenous injection was increased 40-fold than that of naked Ad by the EPR effect (63). Recently, pHPMA was synthesized with insertion of amine reactive carbonyl thiazolidine-2-thione (TT) groups and positively charged quaternary ammonium (QA) groups (64). TT groups take charge of the reactivity function for polymer release from Ad by reductively degradable disulfide bonds under reducing intracellular environment. Also, QA groups provided the increased yield of coating reaction by electrostatic interaction with net negative charge on Ad surface. In this study, multivalent pHPMA containing TT or QA groups showed over 95% reduced binding ability to human erythrocytes, besides the inactivation of naked Ad vectors by interaction with Abs and coagulation factors was protected by polymer-coating. They will potentially cover considerable barriers raised by the viral capsid with little or no effect on virus infectivity.
2.2. Physical engineering by polymers
The surface of Ad contains negative charge due to the structure of external amino acids in the hexon of Ad capsids (65). Non-viral vectors are generally cationic in nature. They include cationic polymers such as poly(ethylenimine) (PEI) and poly(L-lysine) (PLL), cationic peptides and cationic liposomes. Although non-viral vectors are less efficient than viral ones, they have many advantages of safety, simplicity of preparation and high gene encapsulation capability. Based on this property, Ad can form the complex with cationic polymers having the advantage of viral and non-viral vectors.
2.2.1. Cationic polymers
The synthetic cationic polymer (at physiological pH) can be combined with Ad to form a particulate complex, capable of gene transfer into the target cells. Since they are synthetic compounds, many modifications such as molecular weight and ligand attachment can be easily manipulated.
2.2.1.1. Poly(ethylenimine) (PEI)
PEI was used in gene delivery more recently than PLL. It is usually branched with every third amino nitrogen atom being protonated such that PLL has a buffer capacity virtually at any pH value (proton sponge) (66). At the same time, the effect about Ad complex with PEI was reported in two groups (67, 68). They combined the DNA binding property of the cationic polymer PEI and the potent endocytic activity of Ad in a PEI-DNA-Ad complex which provide efficient plasmid delivery. PEI-DNA complexes improved the transduction efficiency of Ad and DNA delivery. At present, branched PEI-25 k showed superior transfection efficiency due to its high density of cationic charge (69). However, high molecular weight PEI induces the strong toxicity.
Many factors affect the efficiency/cytotoxicity profile of PEI complexes such as molecular weight, degree of branching, ionic strength of the solution, zeta potential and particle size (70). Han et al. group recently reported that cross-linking of low molecular weight (LMW) of PEI with a biodegraded linkage could maintain its low toxicity and improve the efficiency of gene delivery (71). They introduced a cationic polymer PDN synthesized by cross-linking PEI-2k with DEG via ester/amide bonds that can be degraded readily under physiological conditions. Gene expression in cells treated with PDN-Ad complex was shown 2- to 6-fold greater than that induced by naked Ad in CAR deficient and CAR over-expressing cell lines. In addition, when 10 μg/mL of polymer PDN cross-linked by low molecular weight (LMW) PEI and diethylene glycol was used for Ad complex, cell viabilities remained more than 75% compared with that (50%) of 25 k PEI. Also, PDN/Ad-SDF1 complex elicited greater anti-tumor activity than these of 25L PEI/Ad-SDF1a or Ad-SDF1a in aggressive LLC tumor model.
2.2.1.2. poly(L-lysine) (PLL)
PLL polymers are one of the first cationic polymers employed for gene transfer (72, 73). They are linear polypeptides with the amino acid lysine as the repeat unit; thus, they possess a biodegradable nature. Fasbender et al. group reported that the complex of Ad and cationic polymers including PLL increased Ad uptake and transgene expression in cells than that in cells treated with Ad alone (74). Also, this paper described well about characterization of the Ad complex with cationic polymers. First, cationic polymers are coated on Ad surface; second, excessive amounts of cationic polymer produce aggregation, which could decrease the efficiency of gene transfer; third, cationic polymers cause Ad to bind to and infect cells through pathways other than the CAR-mediated pathway. This property is very useful for in vivo applications. PLL complexes are, however, rapidly bound to plasma proteins and cleared from the circulation (75). PLL at high concentrations elicit undesirable cytotoxicity. Their applications in vivo are greatly hampered due to their poor biocompatibility and rapid degradation (76). So, to increase transfection efficiency and circulation half-life of Ad complex with PLL, it was modified with PEG. Recent report showed that two types of PEG-grafted PLL had lower toxicity than high molecular weight 24 K PLL (77). Also, the transgene expression in human mesenchymal stem cells treated with PLL-PEG-Ad complex was observed 4.3 fold higher than that of naked Ad.
2.2.1.3. Cationic Lipids (liposomes)
Liposomes are one of the most studied non-viral vectors. They include a group of positively charged lipids at the physiological pH and interact with the negatively charged DNA through electrostatic interaction. Cationic lipids were used mainly in the form of liposomes. In aqueous media, cationic lipids are assembled into a bilayer vesicular-like structure (liposomes). Early Chillon's group used cationic lipid to couple PEG to the virus noncovalently as one way to protect virus from neutralizing antibody (78). They formed complexes using cationic lipid GL-67 and DOPE-PEG. In the optimized ratio of GL-67 to DOPE-PEG, the transgene expression in cells treated with Ad complexed with GL-67/DOPE-PEG in the presence of immune plasma was assessed 50-fold higher than that in cells treated with Ad alone. Also, the transgene expression of cationic lipid GL-67/DOPE-PEG-Ad complex intratracheal administered in immunized mice for lung delivery was 80-fold greater than obtained with Ad alone. These results suggest the feasibility of developing a system in which the virus is effectively shielded from neutralizing antibodies and capable of repeat administration.
Another cationic lipids utilized in the formation of self-assembled Ad complexes is to use several components the following: dimyristoyl phosphatidylcholine (DMPC), cholesterol (Chol), DOTAP [(1,2-dioleoyloxypropyl)-N,N,N-trimethylammonium chloride], 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) (79). This paper engineered 4 different types of artificially enveloped Ad complexes using cationic, neutral, fusogenic, and PEGylated lipids to form the envelopes around nonenveloped virus by self-assembly and obtained extended blood circulation times following intravenous administration and reduced vector immunogenicity. Moreover, the transgene expression of the PEGylated lipid-enveloped Ad after systemic administration was reduced more than 100-fold in the liver and spleen as compared to Ad alone. Also, artificial envelopment led to preferential Ad gene transfer to solid tumors. This indicates that it is possible to target enveloped Ad to tumors via EPR effect.
2.2.1.4. Natural polymer, chitosan
Chitosan, obtained by deacetylation of chitin, is non-toxic, cationic and biodegradable polysaccharide composed of two subunits, D-glucosamine and N-acetyl-D-glucosamine (73). Kawamata Y et al. examined the ability of chitosan to enhance the infectivity of adenovirus to mammalian cells (80). In this study, enhanced effect of chitosan on the infectivity of adenoviruses was observed in the cells that do not express CAR among several cell lines with variable levels of Ad infectivity, indicating the CAR-independent mechanisms for this enhancement effect. These studies suggest chitosan may be a potential candidate for a non-viral vector to safely increase Ad infectivity to mammalian cells.
More in detail possibility of Ad as a systemic injection agent was showed by Park's group (81). In this study, they generated Ad complex based on chitosan and PEG through electrospinning. In addition, Ad-mediated tumor-targeting vector for systemic administration was developed by conjugating folic acid (FA) to chitosan-PEG for tumor cells expressing folate receptor. The transduction efficiency of Ad/chitosan-PEG-FA at 20% FA ratio complex was observed around 2.8- or 5.3-fold higher than that of naked Ad or Ad/chitosan-PEG, respectively. Moreover, to examine the induction of immune response against Ad, the IL-6 cytokine level induced in macrophage cells treated with Ad/chitosan-PEG-FA was reduced 2.7-fold lower than that of the cells treated with naked Ad. These results suggest that Ad nanocomplex based on chitosan with tumor target moiety can be used effectively with reduction in the risk of side effects associated with low immune responses against Ad for cancer therapy.
2.2.1.5. Bioreducible polymers
The currently available cationic polymers have significant acute toxicity concerns such as cellular toxicity, aggregation of erythrocytes, and entrapment in the lung capillary bed (82), and mostly due to their poor biocompatibility and non-degradability under physical conditions. Also, for successful transgene expression, intracellular barriers have to be overcome. After intracellular uptake of polyplexes into endosomal vesicles, it has to escape from endosome for gene delivery toward the nucleus (83). However, although the proton sponge mechanism of PEI is believed to contribute to endosomal release (84), the ability of cationic polymers to escape from endosome is still insufficient.
To develop more intelligent polymers, disulfide bonds were introduced in the design and synthesis of biodegradable polymers (85). Disulfide bonds are more hydrolytically stable than ester bonds in the extracellular environment and can be cleaved rapidly by glutathione and thioredoxin reductase in cytoplasm (85-88), resulting in rapidly released from polyplexes to mediate efficient gene expression. Also, cytotoxicity of polymers including disulfide bond was decreased because it can be degraded into non-toxic small molecules in cells. We introduced arginine-grafted bioreducible polymer (ABP) as grafted arginine residue in backbone structure having disulfide bonds (89). The enhanced transductional effect on Ad complex formed with ABP via physical interaction polymer showed by our group (90). In our study, Ad surface was physically coated by cationic ABP polymer that was confirmed by TEM and DLS results. The enhanced transgene expression was observed in variable cells treated with ABP-coated Ad complex, with minimal toxicity compared with 25 k PEI and lower immunogenicity.
For more closely approach of clinical setting, our group, also, generated oncolytic Ad complex chemically conjugated with the cationic ABP polymer for hepatoma-specific therapy via systemic administration (91). This paper showed in vitro and in vivo safe profiles in details about the combination of oncolytic Ad and the cationic/bioreducible polymer. The combination of liver cancer-specific self-replicating oncolytic Ad and the cationic ABP polymer via chemical conjugation led to the reduced interaction with human serum proteins and the ability to avoid the innate and adaptive immune response by shielding the immunogenic sites of Ad surface. Moreover, surface modification of Ad via chemical conjugation with ABP polymer showed the improved safe profiles and enhanced efficacy to overcome the obstacles of Ad such as short half-life, Ad-associated liver toxicity and the induction of strong immunity against Ad for in vivo application.
Most of non-viral systems used to overcome in vivo barriers of Ad reduce the transduction efficiency of Ad. However, we reported positive results providing proof of concept that systemic targeting is possible (92). In this study, biodegradable poly(cystaminebisacrylamide-diaminohexane) [poly(CBA-DAH)] (CD) was formed complex with oncolytic Ad by physical charge interaction. Moreover, RGD peptide as an active targeting moiety was conjugated to the bioreducible CD polymer connected with polyethylene glycol (PEG). The results of much enhanced cytopathic effect and strong induction of apoptosis of Ad/CD-PEG500-RGD in human cancer cells expressing integrins for RGD targeting, regardless of CAR expression suggested that RGD-conjugated bioreducible polymer might be used to deliver oncolytic Ad safely and efficiently for tumor therapy.
2.2.2. Encapsulation
Alginate- and poly(lactic-co-glycolic acid) (PLGA)- based biodegradable microparticles have been investigated for sustained and targeted/localized delivery of agents such as purified protein, bacteria, DNA or viruses (93-96). The use of biodegradable PLGA and alginate microparticle to encapsulate adenoviral vector provides protection from preexisting humoral immune responses and guard viral vectors from neutralizing antibody responses. Since alginate microspheres are biodegradable and no harsh treatments, the therapeutic activity of adenoviral vectors in the microparticles was maintained (97). Encapsulation of adenoviral vector into alginate microparticles could effectively eluded the virus-specific immune response against pre-existing immunity (98). Moreover, Ad encapsulated in the alginate gel not only was released in a sustained manner, but was maintained the transduction efficiency of Ad vector for a prolonged period in vitro, resulting in preserving biological activity of Ad (99).
PLGA is relatively nontoxic, and in vitro and in vivo degradation occurs by acid or base catalyzed hydrolysis. However, when labile protein or drugs are microencapsulated within PLGA microspheres, they are prone to degrade or aggregate during the harsh formulation conditions, including the exposure to an aqueous/organic interface. Dr. Park's group used PEG to protect the degeneration of Ad during the encapsulation of Ad in PLGA reported (100). PEGylated Ad exhibited the enhanced physical stability against harsh formulation conditions, such as high shear stress and low pH, during encapsulation within PLGA microspheres. PLGA microspheres encapsulating PEG-Ad showed enhanced transgene expression and reduced immune response in vitro.
These encapsulation methods of Ad in biodegradable polymers can induce long-term therapeutic effect by sustained release from Ad-encapsulated particles around target site. Microparticles containing Ad will be delivered by various routes without any obvious side-effects.
3. Active targeting
Since many solid tumors possess leaky vasculature that is hyperpermeable to macromolecules, the retention of adenovirus coated with the polymers like PEG or pHPMA within solid tumors and tumor stroma can be achieved (EPR effect) (41). Most of efforts to develop synthetic polymers for coating Ad have been focused on the following aims; blocking receptor-mediated infection of hepatocytes (low liver toxicity) and phagocytic scavenging by kupffer cells (clearance in liver), evading neutralizing antibody against Ad (clearance in blood), prolonging circulation time in blood, enhancing EPR effects (101, 102). Despite the use of the mentioned polymers above for delivering Ad vectors in vivo has significantly reduced innate and adaptive immune responses, attaching targeting moiety to the ends of polymers coated Ads is inescapable reality for successful cancer therapy. The reason is that sufficient therapeutic efficacy is not able to be achieved only with passive accumulation of shielded Ad with polymers because of the reduced transduction efficacy due to the natural stealth effects of polymer itself (43, 103). In this part, we will more focus on the current advances in targeting of Ad gene delivery via polymers including tumor-targeting ligands. In order to shield Ad vectors from recognition by host immune systems and address the virus to tumor cells, several kinds of polymers as mentioned above have been used for coupling with tumor-homing ligands, which showed the great potentials for cancer therapy in vivo as well as in vitro. So far, targeting ligands for addressing Ad/polymer conjugates have been utilized with 4 different categories as following (Table 2).
Table 2. Classification of targeting ligands linked to the Ad/polymers for active targeting to tumor cells.
| Ligand type | Targeting ligand | Polymer linker | Ad type | Target cell types and comments | Antitumoral effect | Ref. | |
|---|---|---|---|---|---|---|---|
| Homing peptides | linear RGD | PEG | Ad-ΔE1/Luc | CAR (-/+) cells | No | (114) | |
| Ad-ΔE1/Luc | CAR (-/+) cells | No | (115) | ||||
| cyclic RGD | Ad-ΔE1/IκB | Down regulation of E-selectin, ICAM-1, VCAM-1, IL-6, IL-18, VEGF A and Tie-2 | No | (117) | |||
| Ad-ΔE1/GFP-Luc | CAR (-/+) cells | No | (116) | ||||
| Ad-ΔE1/GFP-Luc | In vitro & in vivo bioluminescence imaging | No, but tumor imaging | (118) | ||||
| PEG-CBA-DAH | Oncolytic Ad (Ad-ΔB7-U6shIL8) | CAR (-/+) cells and oncolytic effects | No | (90) | |||
| SIKVAV | pHPMA | Ad-ΔE1/Luc | α6β1 integrins-positive Prostate cancer cells | No | (119) | ||
| SIGYPLP | Ad-ΔE1/Luc | HUVECs | No | (120) | |||
| CGKRK | PEG | Ad-ΔE1/HSV-tk | antitumor effects against primary tumors and metastases | Yes | (121) | ||
| Growth factors | EGF | PEG-PCI | Ad-ΔE1/β-gal | EGFR (-/+) & CAR (-/+) cells | No | (126) | |
| pHPMA | Ad-ΔE1/Luc or Ad-wt | EGFR (-/+), A549, A9, A9-EGFR And SKOV-luc tumor (i.p.) | Yes, and tumor imaging | (127) | |||
| PEG-biotin | Avidin-modified Ad-ΔE1/GFP | EGFR (-/+), A431 (+) and MCF7 (-) | No | (128) | |||
| bFGF or VEGF | pHPMA | Ad-ΔE1/β-gal, Ad-ΔE1/GFP | A9, A9-21, HUVE, A549 | No | (52) | ||
| FGF2 | PEG | Ad-ΔE1/Luc or Ad-wt | FGF (-/+), SKOV3 tumors (i.p.) | Yes | (133) | ||
| Ad-ΔE1/β-gal | In vivo transduction (SKVO3, i.p.) | No | (132) | ||||
| Antibodies | EGFR | Cetuximab | pHPMA | Ad-ΔE1/Luc or Ad-wt | EGFR (+) cells but no EGFR phosphorylation | Yes | (146) |
| Herceptin | PEG | Ad-ΔE1/GFP | Herceptin (-/+) cells | No | (141) | ||
| Ad-ΔE1/GFP, oncolytic Ad (DWP418) | Herceptin (-/+) cells, and tumor models of EGFR (+) SKOV3, MDA-MB435 / EGFR (-) MCF7-mot | Yes | (142) | ||||
| E-selectin | pHPMA | Ad-ΔE1/Luc | TNF-α-activated HUVECs | No | (150) | ||
| PEG | Ad-ΔE1/GFP-Luc | TNF-α-activated HUVECs | No | (116) | |||
| PSMA | pHPMA | AΔ-ΔE1/Luc | PSMA-positive LNCaP | No | (151) | ||
| Natural source | Folate | PEG | Ad-ΔE1/GFP | Many cancerous cells | No | (163) | |
3.1. Homing peptides
The concept of vascular zip codes is well known strategy to find and develop numerous tumor homing peptides (104). A variety of cancer cell binding and/or penetrating peptides have been found and utilized for biological delivery of drugs and nanoparticles to tumor-specific vessels other than blood vessels (105). Among them, RGD peptide is most frequently being used for endowing targeting moiety to Ad/polymer complexes.
3.1.1. RGD
In general, endothelial cells are active participants in varieties of diseases including cancer (106) and chronic inflammation such as rheumatoid arthritis (107). Many kinds of target epitopes overexpressed on activated angiogenic- or proinflammatory-endothelial cells have been identified, including av-integrins (108), E-selectin (109) and vascular endothelial growth factor receptors (110). It is well-known that Arg-Gly-Asp (RGD) peptide binds with high affinity to av-integrins that are overexpressed on angiogenic blood vessels (111). The peptide ligands containing the Arg-Gly-Asp (RGD) triad, which show good endocytotic ability, strong affinity and selectivity to the many kinds of integrins, have been widely developed and utilized to target the tumor-associated cells expressing the integrins receptors (112, 113). Technically, the RGD ligand can specifically recognize and bind with integrin receptors, which are important biomarkers overexpressed in sprouting tumor vessels and most tumor cells (111).
Ad coated with PEG decorating the linear type of RGD peptide on the end of PEG showed high transduction efficiency and Ab evasion ability (114, 115). They constructed that replication incompetent Ad expressing firefly luciferase (Ad-ΔE1/Luc) was covalently attached to PEG (3.4 k) linked to linear type of RGD peptide (YGGRGDTP). In B16BL6 (CAR-negative) cells, The RGD-PEG-Ad showed 60-fold and 200-fold higher transgene expression rates than naked Ad and PEG-Ad, respectively (114). In the presence of anti-Ad antibody, RGD-PEG-Ad retained more than one-tenth of its transduction efficiency, whereas Ad-RGD containing an RGD peptide in the HI loop of the fiber lost more than 99% of its activity in the presence of Ab.
For retargeting Ad with the same strategy but using cyclic RGD (cRGD) peptide, bifunctional PEG was conjugated onto the viral capsid of Ad encoding luciferase as a reporter gene and cRGD peptide was then introduced to the other functional group of the PEG molecule (116). The RGD-conjugated Ad (Ad-TL-PEG-RGD) exhibited a relatively high transduction in HUVEC cells when compared with that of AdTL-PEG-Cys without RGD peptide. The transduction efficiency of Ad-TL-PEG-RGD was significantly reduced by the presence of RGD-modified protein, but not by recombinant knob 5. They also published another result using the same RGD-PEG-modified Ad encoding dominant negative lκB (dnlκB) as a therapeutic gene (117). Since a dnlκB that contains serine-to-alanine mutations at amino acids 32 and 36 can blocks endogenous lκB phosphorylation and subsequent proteosome-mediated degradation, thereby inhibiting NF-κB mediated gene expression, TNF-α driven expression of all pro-inflammatory and pro-angiogenic genes (E-selectin, ICAM-1, VCAM-1, IL-6, IL-8, VEGF-A and Tie-2) was completely abolished in HUVECs transduced with the RGD-targeted Ad delivering the dnlκB gene via αvb3. The cRGD-modified Ad vector showed significantly enhanced transduction efficiency of integrin-positive tumors than naked Ad through intranvenous administration that was exhibited using in vivo bioluminescence tumor imaging (118). As polymer carriers showing low cytotoxicity, high transduction efficiency and characteristic of active-targeting to cancer cells expressing αv-integrins, the cRGD-conjugated bioreducible polymer (CD-PEG-RGD) delivering for delivery of oncolytic Ad expressing shRNA against IL-8 mRNA, was introduced by our group (92). The oncolytic effects of Ad coated the polymer were dramatically enhanced, especially in cancer cells expressing high levels of αv-integrins. Not only that, the expression levels of IL-8 and VEGF were significantly suppressed in the cancer cells treated with Ad coated with the polymers when compared with the results by naked Ad. No prominent cell-killing effects and down-regulation of IL-8 and VEGF expression were observed in human normal cells.
3.1.2. Others
With the aim of addressing Ad vectors to prostate cancer cells via alpha6beta1 integrins, whose expression if upregulated during prostate cancer progression, transduction efficiency of Ad vectors was enhanced in PC-3 cells by coating with pHPMA linked with alpha6-integrin binding peptide (-SIKVAV-) derived from laminin (119). The retargeted viruses were delivered to and expressed reporter proteins in the tumor with reduced liver tropism and slower plasma clearance. Parker et al. also reported the capability of another homing peptide (SIGYPLP) for incorporating on the polymer-modified Ad vectors, which is the cell binding hepapeptide to enhance transgene expression (120). The levels of transgene expression by the retargeted Ad vectors with the homing peptide were enhanced to greater than 15 times in HUVECs that observed using naked viruses. By conjugating CGKRK tumor vasculature homing peptide to the end of a 20-kDa PEG chain, tumor vascular targeted delivery of Ad vector expressing reporter protein or HSV-tk protein was accomplished successfully, resulting in much higher transgene expression in tumor or superior antitumor effects against primary tumors and metastasis than that of naked Ad and Adv-PEG, respectively (121).
3.2. Growth factors
Growth factors are capable of stimulating cellular growth, proliferation and cellular differentiation and play important role for regulating a variety of cellular processes. They typically act as signaling molecules between cells and often promote cell differentiation and maturation. Abnormality in the growth factor signaling pathways can lead to uncontrolled growth and development (122). Cancer has been now recognized to be the result of a multistep process, the unregulated expression of growth factors or components of their signaling pathways is the one of the critical phenomena in malignant transformation. With this reason, many growth factors have been frequently utilized as targeting moieties because they can bind to specific receptors overexpressed on the surface of many tumor cells and associated with an aggressive tumor phenotype. Among them, endothelial growth factors (EGF) and fibroblast growth factors (FGF) have been used as attractive targeting ligands for retargeting of Ad (123, 124).
3.2.1. EGF
EGF is a low-molecular weight polypeptide (6.0 k) and plays an important physiological role in the regulation of cell growth, proliferation and differentiation by binding to its receptor, the epidermal growth factor receptor (EGFR). The EGFR (also known as ErbB1), a family of receptor tyrosine kinase proteins, is a promising tumor-associated target, overexpressed in more than 60% of ovarian cancers and associated with the malignant phenotype when compared to normal cells (125). The strategies about targeting of polymer-coated Ad to the EGFR have been recently reported by three groups (126-128).
Berg et al. reported that photochemically enhanced transduction with EGFR-targeted and polymer-complexed Ad was accomplished by the binding of biotin-EGF to the polymer poly(2-(dimethylamino)ethyl methacrylate) (pDMAEMA)-avidin. pDMAEMA-avidin effectively enhanced transduction through unspecific Ad-uptake into cells, while pDMAEMA-PEG provided charge shielding of the Ad-polymer complexes and increased the specificity to EGFR when biotin-EGF ligands were used (126). Interestingly, they found that a physical targeting technology, termed photochemical internalization (PCI), significantly enhanced transduction efficiency with untargeted polycation-coated Ad vectors without any targeting ligands (129). The endocytic compartments in cells can be disrupted by light activation of photosensitizing compounds that localize in the endocytic vesicles (130). The selective transduction was confirmed that the transduction of EGFR-targeted viral complexes connected with PEG was inhibited by 66% in CAR-deficient cells and by 47% in CAR-expressing cells in receptor antibody experiments. In this study, the combinatorial approach with using biological and physical methods is capitally noted for specific and efficient delivery of Ad.
Using the similar concept of biotin-avidin interaction as mentioned, avidin-modified Ad (Ad-avi) was introduced to immobilize biotin-PEG-EGF conjugated (128). In their study, more conveniently, EGF-conjugated to the distal end of a PEG chain with biotin can be easily complexed with avidin-conjugated on the surface of Ad by high affinity of biotin-avidin interaction. The transduction efficiency of Ad-avi/biotin-PEG-EGF complexes was dramatically enhanced with increasing the amount of biotin-PEG-EGF conjugate in A431 cells over-expressing EGFR. However, there was no significant difference in transgene expression level for MCF7 cells lacking EGFR. To prove whether transduction pathway of Ad-avi/biotin-PEG-EGF would be mediated through EGF targeting moiety, treatment of free EGF successfully blocked the GFP expression levels of Ad-avi/biotin-PEG-EGF.
The selective/enhanced transduction and the therapeutic efficacy of murine EGF (mEGF)-conjugated pHPMA retargeted Ad were evaluated by Seymour groups (127). To achieve tumor-selective infection and therapy as well, mEGF peptide, for subsequent coating of Ad, was conjugated to HPMA copolymer. The ability of mEGF-conjugated pHPMA to stimulate EGFR tyrosine kinase activity was determined by EGFR autophosphorylation using Western blotting. The tropism of Ad expressing luciferase was ablated by HPMA polymer and retargeted by mEGF peptide by luciferase assay compared to the unmodified Ad in cancer cell types used. Not only oncolytic Ad, even wild-type Ad (Ad5WT) replicates more quickly in many human cancer cells than in human normal cells, antitumoral effects of Ad5WT coated with untargeted pHPMA or targeted mEGF-pHPMA conjugates was evaluated in murine models bearing human ovarian tumors. mEGF-pHPMA-Ad5WT mediated a significant longer median survival compared with PBS (34 days with PBS versus 64.5 days with mEGF-pHPMA-Ad5WT) and untargeted pHPMA-Ad5WT (46 days with pHPMA-Ad5WT) versus 64.5 days with mEGF-pHPMA-Ad5WT).
3.2.2. FGF
FGF family of growth factors has shown variable actions including their effect on cellular proliferation, angiogenesis and neurotrophic effects. Many studies have reported that the receptors of FGFs were correlated and highly expressed in cancers to more aggressive tumors with a greater tendency to metastasis (131).
The first trial of polymer-coated Ad with targeting moiety was achieved by Fisher et al (52). They developed the covalent coating and retargeting strategy for safe and efficient delivery of Ad using a multivalent hydrophilic polymer based on pHPMA. As targeting ligands, basic FGF (bFGF) or VEGF was incorporated on to the polymer-coated Ad that showed CAR-independent binding and uptake into cancer cells expressing appropriate receptors. Even though Ads expressing GFP proteins were chemically shielded with targeted polymers (bFGF-pHPMA), transduction efficiency of bFGF-pHPMA-Ad in A549 cells expressing FGF receptors was quite similar with that of naked Ad, while untargeted Ad/polymer (pHPMA-Ad) gave no detectable fluorescent signal. In primary human embilical endothelial (HUVE) cells having low levels of CAR but expressing receptors for both VEGF and bFGF, more efficient GFP expression was detected using targeted polymers/Ads (VEGF-pHPMA-Ad or bFGF-pHPMA-Ad) than that of naked Ad. Respective ligand specificity of each Ad/polymers was also confirmed by competition assay using antibodies against bFGF or VEGF. This report firstly demonstrated that targeted polymer can permit efficient and selective retargeting of Ad and evade neutralizing antibodies against Ad.
Using heterofunctional PEG molecules decorating FGF homing peptide to retargeting Ad vectors to cancer cells expressing FGF receptors, the immunological properties such as protection from neutralization and cytokine secretion of targeted polymer-coated Ad were noted both in vitro and in vivo (132). In contrast with modification technology of Ads with monofunctional PEG, heterofunctional PEG molecules allow the further covalent modification of the capsid with targeting molecules, which serves to overcome the limit of inefficient gene transfer into specific cell types such as cancer cells. As well as, it can ablate the normal tropism of the virus, reduce transduction of nontarget tissues in vivo and prolong blood circulation time. The enhancement in transduction was 10-fold higher than naked Ad or Ad/PEG without FGF-targeting moiety in SKOV3.ip1 cells expressing FGF receptors abundantly that was dependent on the binding of the coupled FGF to its high-affinity receptor and is not dependent of CAR on the surface of the cells. The retargeted vectors successfully escaped from neutralizing antibodies in the presence of human anti-Ad neutralizing serum, generating humoral and cellular immune responses against capsid proteins of Ad as well.
Fisher et al. also reported detargeting by HPMA polymer followed by retargeting by FGF2 peptide of Ad5 (133). In contrast with their previous report (52), they introduced improved polymer coating strategies, accomplishing target cell-selectivity ratios of up to five thousand, and with complete inhibition of hepatic infection in vivo. The optimal condition of the coating polymer concentration (2 to 20 mg/ml) was evaluated to ablate natural tropism of Ad by polymer coating as much as possible, while PEGylation has been reported that it does not abrogate liver transfection following intravenous injection (134, 135). Transduction efficacy of Ad was dramatically decreased a further 1000-fold when the polymer concentration was increased to 20 mg/ml minimizing unwanted infection of non-target cells. By imposing FGF2-targeting peptide on pHPMA-Ad, both naked Ad and FGF2-pHPMA-Ad generated a characteristic cytopathic effect (CPE) showing 1000-fold higher cancer cell-killing effect than untargeted-pHPMA-Ad. The FGF-targeted pHPMA-Ad showed extended blood circulation levels relative to the untargeted pHPMA-Ad or naked Ad (21% versus 12% or 1% after 30 min, respectively). Intraperitoneal (i.p.) administration of polymer coated-Ad (pHPMA-Ad) without targeting moiety had little effect on the progression of cancer, while the FGF-targeted Ad/polymer resulted in a substantial decrease in tumor burden. This report also emphasized the importance of targeting ligand for efficient systemic delivery of Ad for cancer therapy, since stealing Ad particles with only polymers will not be fulfilled for sufficient therapeutic outcome.
3.3. Antibodies
Targeted antibody therapy has been employed to treat many forms of cancer including non-Hodgkin's lymphoma (136), colorectal cancer, head and neck cancer and breast cancer (137). By utilizing biologically specific interactions such as Ag-Ab binding, active targeting can be achieved to increase the efficacy of drug delivery to the target cells. Polymers is engineered by means of Ab coupling according to the biological characteristics of tumor tissues overexpressing cell surface tumor-associated antigens that are at low levels in normal tissue cells. Specific interactions between each Ab with its Ag exposed on target tissues causes the selective accumulation of Ab-targeted drug in the target tissue. The advantage of active targeting is decrease of adverse side effects, since targeted materials can accumulate only in the tumor sites, relatively. The strategy for retargeting of Ad gene delivery with polymer is being focused on tumor specific Ab such as Trastuzumab (also known as Herceptin)/Cetuximab or MHES against EGFR or E-selectin, respectively, due to high specificity of Ab for the extracellular domain of its receptor. Additionally, it is assumed that expression levels of tumor specific receptors could directly lead to a predictive biomarker for response to Ab targeting, therapy as well.
3.3.1. EGFR
Herceptin is a humanized monoclonal Ab that interferes with the HER2/neu receptor (HER2) that is being studied for use with many cancers (138). The HER2 gene (also known as HER2/neu and ErbB2 gene) is overexpressed in 20-30% of early-stage breast cancers (139). It has been also used with some promising success in women with uterine papillary serous carcinoma overexpressing HER2/neu (140). Utilizing Trastuzumab (anti-HER2/neu monoclonal Ab, HER), retargeted Ad with a spacer of PEG (HER-PEG-Ad) was reported by our group that showed significantly enhanced transduction efficiency in HER2/neu positive cells (human breast cancer cells) when compared to PEG-Ad with reduced innate immune response to resolve the problem of immunogenicity of naked Ad (141). We further demonstrated that reduced both innate and humoral immune responses and prolonged blood circulation time of Ad coated with HER-PEG showing cancer-killing effects (142). DWP418, the oncolytic Ad expressing relaxin and showing enhanced tumor-killing effect (11, 17, 29), decorated with HER-PEG was successfully targeted to tumors actively via HER and suppressed tumor growth in mouse xenograft model of human ovarian cancers (SK-OV3 and MDA-MB435: Her2/neu positive cells). However, only mild antitumoral activity of DWP418-PEG-HER was assessed in Her/neu negative MCF7-mot tumors with the similar tumor-regression effect by untargeted DWP418-PEG, still showing higher antitumoral effect that might be caused of prolonged blood circulation time against innate & humoral immune responses. The tumor to liver bioaccumulation ratio of Trastuzumab-conjugated DWP418 (DWP418-PEG-HER) was 1010-fold greater than naked DWP418 and a thousand times greater than detargeted DWP418 (DWP418-PEG). The data exactly and fully reflect the strong potential of retargeted oncolytic Ad coated with polymer as a therapeutic vector for systemic administration, with characteristics of replicating in, producing progeny Ads and ultimately killing cancer cells, without any side effects such as liver toxicity and infection to unwanted normal cells even though the replication of DWP418 is restricted in human normal cells as described (17, 29).
Cetuximab (IMC-C225, Erbitux), anti-EGF receptor Ab, is also a humanized monoclonal Ab and many clinical trials have been conducted to investigate its efficacy for treatment of epithelial cancers, including metastatic colorectal cancer (143), ovarian carcinoma (144, 145). Using this selective interaction between Cetuximab and EGFR highly expressed in many kinds of malignant tumors when compared to normal cells (125), Cetuximab was utilized to retarget Ad transduction of EGFR-positives in vitro and in vivo (146). They demonstrated that pHPMA copolymer coating and covalent attachment of Cetuximab successfully retargeted Ad to the cells expressing EGFR. Covalent coating of Ad with Cetuximab-targeted HPMA polymer was clearly able to deliver Ad infection via the EGFR, with no evidence of receptor tyrosine kinase activation. Interestingly, Cetuximab was found to promoter receptor-mediated internalization without autophosphorylation of EGFR which can induce mitogenic response. Retargeting of wild-type Ad with Cetuximab-targeted polymer prolonged survival in an i.p. model of human ovarian cancer, while only Ad/polymer showed the similar tumor burden with PBS-treated mice.
3.3.2. E-selectin
E-selectin is a cell adhesion molecule expressed only on endothelial cells activated by cytokines like IL-1 and tumor necrosis factor (TNF)-α by damaged cells induces the over-expression of E-selectin on endothelial cells of nearby blood vessels. It also plays an important part in inflammation and is being used as an emerging biomarker for the metastatic potential of some cancers including colorectal cancer and recurrences (147). With the reason that E-selectin has been known as an attractive endothelial cell surface marker in cancer (147-149), E-selectin-specific Ab for retargeting of polymer-coated Ad was reported (116, 150). Ogawara et al. demonstrated PEGylated Ad vectors exposed E-selectin specific Ab (MES-1) on the end of PEG, which can target to activated endothelial cells (116). It was the first trial using Ab as a targeting ligand with polymer to modify Ad tropism that enhances transgene delivery of Ad to activated vascular endothelial cells. Utilizing bifunctional PEG, Ad vectors were chemically coated with PEG linker (3.4 k), PEGylated virus was directly used in the following coupling reaction with E-selectin Ab (mouse IgG2a anti-human E-selectin). Although PEGylation of Ad completely abrogated Ad-transduction pathway through CAR-knob interaction with exhibiting approximately 10-fold less efficiency than naked Ad, introduction of anti-E-selectin antibody onto the PEGylated Ad restored the similar transduction efficiency with naked Ad. While PEGylation can only increase blood circulation time of Ad, homing selectivity was achieved by decorating with additional anti-E-selectin Ab in TNF-α-activated human umbilical vein endothelial cells (HUVEC) or endothelial cells in the inflamed skin for 3 hours (delayed-type hypersensitivity [DTH] model). Retargeting of Ad using the same type of anti-E-selectin Ab was also achieved as shown above, while Ad was first conjugated with an amino-reactive multivalent hydrophilic polymer based on pHPMA (150). This pHPMA-coated Ad was efficiently taken up into HUVECs stimulated by TNF-α and internalized through a viable receptor-mediated endocytosis pathway via E-selectin and its Ab on Ad/polymer complex.
3.3.3. PSMA
Using a universal bungarotoxin (BTX) linker, non-covalent and non-destructive attachment of a recombinant Ab fragment to pHPMA-modified Ad vectors has been introduced (151). The principle of this strategy is based on the strong and non-covalent interaction between acetylcholine receptors and a-BTX. Recombinant anti-prostate specific membrane antigen (PSMA) Ab scFv fragment containing the BTX binding region (scFv-BTX) was added to the polymer-modified BTX binding peptide (BTXbp)-bearing Ad (Ad-pHPMA-BTXbp) encoding luciferase protein. Transduction efficiency of the targeted Ad vectors was 5 to 10-fold higher than other experimental groups in PSMA-positive LNCaP cells.
3.4. Natural source (Folate)
Folate (the naturally occurring form of folic acid) is the natural source of vitamin B9 found in variable foods and needed to human body to synthesize DNA, repair DNA and methylate DNA (152). It is also important for rapid cell division and growth, especially during infancy and pregnancy, as well as tumor growth (153). It has been implicated with respect to several malignancies, including colorectum, lung and cervix, and may impact prostate cancer (154, 155). Folate receptor overexpressed on the surface of many cancer cells (156), has been made achievements for targeting anticancer agents or imaging agents to the tumor cells using folate-conjugated carriers (157, 158). The report regarding modification of Ad surface with folate conjugated to the distal end of PEG chain (3.4 k) was demonstrated that chemically modified Ad with targeting ligand showed selective transduction efficacy in KB cells overexpressing folate receptors, but not in A549 lacking the receptors. They suggested that folate-Ad/polymer complex showing reduced immune response, might be taken up by KB cells by folate receptor-mediated endocytosis, not by CAR-mediated endocytosis.
Future prospects
There is no doubt that Ad vectors are attractive instruments for cancer therapy, with carrying therapeutic agents or by themselves as virotherapy. Although Ads have many advantages as mentioned, their clinical applications are still restricted due to some problematic factors. For safer and more effective cancer therapy, therapeutic Ads have to be treated to not only primary tumor but metastatic tumor in disseminated tissues throughout the body.
To treat the Ad vectors to metastatic tumor, systemic administration is the most logical route for effective delivery. However, systemic delivery of Ads causes a variety of rapid physiological responses. Once naked Ads are administered intravenously, they are immediately extinguished by the major guarding system such as innate and adaptive immune responses (RBC, macrophage, DC and neutralizing Abs). As well as, Ads are accumulated in liver through hepatocyte receptor-mediated endocytosis and quickly removed by Kupffer cells having scavenging effects (Fig. 1A). Thus, Ad vectors are rarely located within tumor sites and worse fact is that lacking of CAR expression for Ad infection is correlated with the development of tumor malignancy (159).
Fig. 1.
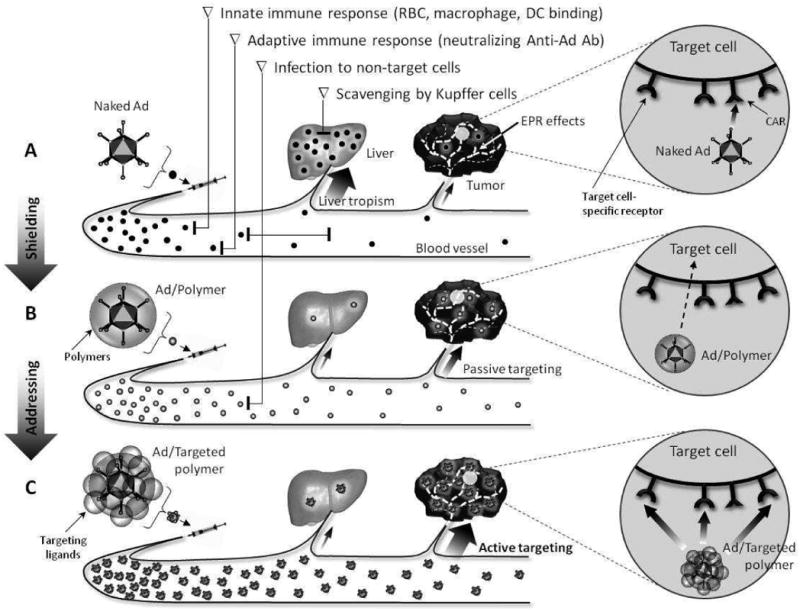
Schematic representation of delivery pathway of systemically administered naked Ad, shielded Ad with polymer (passive targeting) or targeted polymer-coated Ad (active targeting).
With the efforts to evade the immune responses, avoid the toxic side effects and improve the therapeutic efficacy, numerous challenges have been tried so far. Even though direct modifications of the Ad-fiber gene well known as major immunogenic site have been accomplished well (38), these approaches still induce immune responses and the tropism of Ad was not much changed since hexon proteins are exposed as ever (160, 161).
To improve the pharmacokinetics of systemically administered Ad in vivo, the usage of combination with non-immunogenic constituent such as non-viral system is unable to avoid. Hybrid vector consisting of viral and non-viral components has come to be created. Various synthetic polymers were engineered using the negatively charge characteristics and lysine residues on virus surface via chemical conjugation or physical interaction. As shown in Fig. 1B, administered polymer-shielded Ad vectors can survive more than naked Ads with the following expected reasons: evacuation from the guarding systems in blood system with the loss of immunogenicity; blocking of receptor-mediated infection to hepatocytes and phagocytic scavenging by Kupffer cells; prolongation of circulation time in blood; enhanced accumulation in tumor via EPR effects.
Even if the polymer-modification of Ad vectors is certainly able to overcome the disadvantages of naked Ad after systemic administration, passive accumulation of shielded Ad with any appropriate polymers is not sufficient to achieve successful therapeutic efficacy. It is because that the polymers themselves have no selectivity between normal and tumor cells although oncolytic Ad vectors are participated. In conclusion, endowment of targeting moiety to the ends of polymers coated Ads is inescapable fact for successful cancer eradication. Actively targeted polymer-shielded Ad vectors linked with tumor-targeting ligands can show not only more increased accumulation within tumors, followed by selective capture by target cell-specific receptors and enhanced therapeutic efficiency, but also much reduced undesired transductions and clearance in blood without any fruit (Fig. 1C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953;84(3):570–3. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- 2.Huebner RJ, Rowe WP, Schatten WE, Smith RR, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9(6):1211–8. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–9. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 4.Georgiades J, Zielinski T, Cicholska A, Jordan E. Research on the oncolytic effect of APC viruses in cancer of the cervix uteri; preliminary report. Biul Inst Med Morsk Gdansk. 1959;10:49–57. [PubMed] [Google Scholar]
- 5.Zielinski T, Jordan E. Remote results of clinical observation of the oncolytic action of adenoviruses on cervix cancer. Nowotwory. 1969;19(3):217–21. [PubMed] [Google Scholar]
- 6.Imperiale MJ, Kochanek S. Adenovirus vectors: biology, design, and production. Curr Top Microbiol Immunol. 2004;273:335–57. doi: 10.1007/978-3-662-05599-1_10. [DOI] [PubMed] [Google Scholar]
- 7.Kanerva A, Hemminki A. Modified adenoviruses for cancer gene therapy. Int J Cancer. 2004;110(4):475–80. doi: 10.1002/ijc.20129. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–6. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 9.Jounaidi Y, Doloff JC, Waxman DJ. Conditionally replicating adenoviruses for cancer treatment. Curr Cancer Drug Targets. 2007;7(3):285–301. doi: 10.2174/156800907780618301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pesonen S, Kangasniemi L, Hemminki A. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm. 2011;8(1):12–28. doi: 10.1021/mp100219n. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Cho JY, Kim JH, Jung KC, Yun CO. Evaluation of E1B gene-attenuated replicating adenoviruses for cancer gene therapy. Cancer Gene Ther. 2002;9(9):725–36. doi: 10.1038/sj.cgt.7700494. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kim JH, Choi KJ, Kim PH, Yun CO. E1A- and E1B-Double mutant replicating adenovirus elicits enhanced oncolytic and antitumor effects. Hum Gene Ther. 2007;18(9):773–86. doi: 10.1089/hum.2006.167. [DOI] [PubMed] [Google Scholar]
- 13.DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61(20):7464–72. [PubMed] [Google Scholar]
- 14.Kim J, Lee B, Kim JS, Yun CO, Kim JH, Lee YJ, et al. Antitumoral effects of recombinant adenovirus YKL-1001, conditionally replicating in alpha-fetoprotein-producing human liver cancer cells. Cancer Lett. 2002;180(1):23–32. doi: 10.1016/s0304-3835(02)00017-4. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Chen Y, Dilley J, Arroyo T, Ko D, Working P, et al. Carcinoembryonic antigen-producing cell-specific oncolytic adenovirus, OV798, for colorectal cancer therapy. Mol Cancer Ther. 2003;2(10):1003–9. [PubMed] [Google Scholar]
- 16.Kurihara T, Brough DE, Kovesdi I, Kufe DW. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106(6):763–71. doi: 10.1172/JCI9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim E, Kim JH, Shin HY, Lee H, Yang JM, Kim J, et al. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum Gene Ther. 2003;14(15):1415–28. doi: 10.1089/104303403769211637. [DOI] [PubMed] [Google Scholar]
- 18.Kwon OJ, Kim PH, Huyn S, Wu L, Kim M, Yun CO. A hypoxia- and {alpha}-fetoprotein-dependent oncolytic adenovirus exhibits specific killing of hepatocellular carcinomas. Clin Cancer Res. 2011;16(24):6071–82. doi: 10.1158/1078-0432.CCR-10-0664. [DOI] [PubMed] [Google Scholar]
- 19.Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18(7):723–7. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 20.Zhang WW, Alemany R, Wang J, Koch PE, Ordonez NG, Roth JA. Safety evaluation of Ad5CMV-p53 in vitro and in vivo. Hum Gene Ther. 1995;6(2):155–64. doi: 10.1089/hum.1995.6.2-155. [DOI] [PubMed] [Google Scholar]
- 21.Shewach DS, Zerbe LK, Hughes TL, Roessler BJ, Breakefield XO, Davidson BL. Enhanced cytotoxicity of antiviral drugs mediated by adenovirus directed transfer of the herpes simplex virus thymidine kinase gene in rat glioma cells. Cancer Gene Ther. 1994;1(2):107–12. [PubMed] [Google Scholar]
- 22.Hirschowitz EA, Ohwada A, Pascal WR, Russi TJ, Crystal RG. In vivo adenovirus-mediated gene transfer of the Escherichia coli cytosine deaminase gene to human colon carcinoma-derived tumors induces chemosensitivity to 5-fluorocytosine. Hum Gene Ther. 1995;6(8):1055–63. doi: 10.1089/hum.1995.6.8-1055. [DOI] [PubMed] [Google Scholar]
- 23.Griffith TS, Anderson RD, Davidson BL, Williams RD, Ratliff TL. Adenoviral-mediated transfer of the TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol. 2000;165(5):2886–94. doi: 10.4049/jimmunol.165.5.2886. [DOI] [PubMed] [Google Scholar]
- 24.Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H, et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 2006;13(13):1010–20. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- 25.Lee YS, Kim JH, Choi KJ, Choi IK, Kim H, Cho S, et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin Cancer Res. 2006;12(19):5859–68. doi: 10.1158/1078-0432.CCR-06-0935. [DOI] [PubMed] [Google Scholar]
- 26.Yoo JY, Kim JH, Kwon YG, Kim EC, Kim NK, Choi HJ, et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther. 2007;15(2):295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- 27.Yoo JY, Kim JH, Kim J, Huang JH, Zhang SN, Kang YA, et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Ther. 2008;15(9):635–51. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 28.Yun CO, Kim E, Koo T, Kim H, Lee YS, Kim JH. ADP-overexpressing adenovirus elicits enhanced cytopathic effect by induction of apoptosis. Cancer Gene Ther. 2005;12(1):61–71. doi: 10.1038/sj.cgt.7700769. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Lee YS, Kim H, Huang JH, Yoon AR, Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006;98(20):1482–93. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 30.Choi IK, Lee YS, Yoo JY, Yoon AR, Kim H, Kim DS, et al. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene Ther. 2010;17(2):190–201. doi: 10.1038/gt.2009.142. [DOI] [PubMed] [Google Scholar]
- 31.Alemany R, Suzuki K, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81(Pt 11):2605–9. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 32.Nemunaitis J, O'Brien J. Head and neck cancer: gene therapy approaches. Part II: genes delivered. Expert Opin Biol Ther. 2002;2(3):311–24. doi: 10.1517/14712598.2.3.311. [DOI] [PubMed] [Google Scholar]
- 33.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10(6):965–76. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3(5 Pt 1):697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Zaiss AK, Colarusso P, Patel K, Haljan G, Wickham TJ, et al. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum Gene Ther. 2003;14(7):627–43. doi: 10.1089/104303403321618146. [DOI] [PubMed] [Google Scholar]
- 36.Kiang A, Hartman ZC, Everett RS, Serra D, Jiang H, Frank MM, et al. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol Ther. 2006;14(4):588–98. doi: 10.1016/j.ymthe.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Molnar-Kimber KL, Sterman DH, Chang M, Kang EH, ElBash M, Lanuti M, et al. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum Gene Ther. 1998;9(14):2121–33. doi: 10.1089/hum.1998.9.14-2121. [DOI] [PubMed] [Google Scholar]
- 38.Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174(11):7179–85. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 39.Oberholzer C, Oberholzer A, Tschoeke SK, Minter RM, Bahjat FR, LaFace D, et al. Influence of recombinant adenovirus on liver injury in endotoxicosis and its modulation by IL-10 expression. J Endotoxin Res. 2004;10(6):393–401. doi: 10.1179/096805104225005832. [DOI] [PubMed] [Google Scholar]
- 40.Seymour LW. Passive tumor targeting of soluble macromolecules and drug conjugates. Crit Rev Ther Drug Carrier Syst. 1992;9(2):135–87. [PubMed] [Google Scholar]
- 41.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- 42.Kreppel F, Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide. Mol Ther. 2008;16(1):16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- 43.Fisher KD, Seymour LW. HPMA copolymers for masking and retargeting of therapeutic viruses. Adv Drug Deliv Rev. 2010;62(2):240–5. doi: 10.1016/j.addr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Kang E, Yun CO. Current advances in adenovirus nanocomplexes: more specificity and less immunogenicity. BMB Rep. 2010;43(12):781–8. doi: 10.5483/BMBRep.2010.43.12.781. [DOI] [PubMed] [Google Scholar]
- 45.Jang JH, Schaffer DV, Shea LD. Engineering biomaterial systems to enhance viral vector gene delivery. Mol Ther. 2011;19(8):1407–15. doi: 10.1038/mt.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70(4):2116–23. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ophorst OJ, Kostense S, Goudsmit J, De Swart RL, Verhaagh S, Zakhartchouk A, et al. An adenoviral type 5 vector carrying a type 35 fiber as a vaccine vehicle: DC targeting, cross neutralization, and immunogenicity. Vaccine. 2004;22(23-24):3035–44. doi: 10.1016/j.vaccine.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Mizuguchi H, Hayakawa T. Targeted adenovirus vectors. Hum Gene Ther. 2004;15(11):1034–44. doi: 10.1089/hum.2004.15.1034. [DOI] [PubMed] [Google Scholar]
- 49.O'Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, et al. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10(8):1349–58. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 50.Myhre S, Henning P, Granio O, Tylo AS, Nygren PA, Olofsson S, et al. Decreased immune reactivity towards a knobless, affibody-targeted adenovirus type 5 vector. Gene Ther. 2007;14(4):376–81. doi: 10.1038/sj.gt.3302875. [DOI] [PubMed] [Google Scholar]
- 51.Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther. 2002;13(15):1887–900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- 52.Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V, Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8(5):341–8. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 53.Mok H, Palmer DJ, Ng P, Barry MA. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther. 2005;11(1):66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Wortmann A, Vohringer S, Engler T, Corjon S, Schirmbeck R, Reimann J, et al. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol Ther. 2008;16(1):154–62. doi: 10.1038/sj.mt.6300306. [DOI] [PubMed] [Google Scholar]
- 55.Hofherr SE, Shashkova EV, Weaver EA, Khare R, Barry MA. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol Ther. 2008;16(7):1276–82. doi: 10.1038/mt.2008.86. [DOI] [PubMed] [Google Scholar]
- 56.Gao JQ, Eto Y, Yoshioka Y, Sekiguchi F, Kurachi S, Morishige T, et al. Effective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administration. J Control Release. 2007;122(1):102–10. doi: 10.1016/j.jconrel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 57.Yao X, Yoshioka Y, Morishige T, Eto Y, Watanabe H, Okada Y, et al. Systemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasis. Gene Ther. 2009;16(12):1395–404. doi: 10.1038/gt.2009.95. [DOI] [PubMed] [Google Scholar]
- 58.Weaver EA, Barry MA. Effects of shielding adenoviral vectors with polyethylene glycol on vector-specific and vaccine-mediated immune responses. Hum Gene Ther. 2008;19(12):1369–82. doi: 10.1089/hum.2008.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1(1):1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kreppel F, Gackowski J, Schmidt E, Kochanek S. Combined genetic and chemical capsid modifications enable flexible and efficient de- and retargeting of adenovirus vectors. Mol Ther. 2005;12(1):107–17. doi: 10.1016/j.ymthe.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki-Kouyama E, Katayama K, Sakurai F, Yamaguchi T, Kurachi S, Kawabata K, et al. Hexon-specific PEGylated adenovirus vectors utilizing avidin-biotin interaction. Biomaterials. 2011;32(6):1724–30. doi: 10.1016/j.biomaterials.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 62.Green NK, Herbert CW, Hale SJ, Hale AB, Mautner V, Harkins R, et al. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11(16):1256–63. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- 63.Fisher KD, Green NK, Hale A, Subr V, Ulbrich K, Seymour LW. Passive tumour targeting of polymer-coated adenovirus for cancer gene therapy. J Drug Target. 2007;15(7-8):546–51. doi: 10.1080/10611860701501014. [DOI] [PubMed] [Google Scholar]
- 64.Subr V, Kostka L, Selby-Milic T, Fisher K, Ulbrich K, Seymour LW, et al. Coating of adenovirus type 5 with polymers containing quaternary amines prevents binding to blood components. J Control Release. 2009;135(2):152–8. doi: 10.1016/j.jconrel.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 65.Karlin S, Brendel V. Charge configurations in viral proteins. Proc Natl Acad Sci U S A. 1988;85(24):9396–400. doi: 10.1073/pnas.85.24.9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci. 1995;92(16):7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker A, Saltik M, Lehrmann H, Killisch I, Mautner V, Lamm G, et al. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther. 1997;4(8):773–82. doi: 10.1038/sj.gt.3300471. [DOI] [PubMed] [Google Scholar]
- 68.Meunier-Durmort C, Grimal H, Sachs LM, Demeneix BA, Forest C. Adenovirus enhancement of polyethylenimine-mediated transfer of regulated genes in differentiated cells. Gene Ther. 1997;4(8):808–14. doi: 10.1038/sj.gt.3300450. [DOI] [PubMed] [Google Scholar]
- 69.Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16(8):1273–9. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 70.Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, et al. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release. 2003;89(1):113–25. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 71.Han J, Zhao D, Zhong Z, Zhang Z, Gong T, Sun X. Combination of adenovirus and cross-linked low molecular weight PEI improves efficiency of gene transduction. Nanotechnology. 21(10):105106. doi: 10.1088/0957-4484/21/10/105106. [DOI] [PubMed] [Google Scholar]
- 72.Wu GY, Wu CH. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987;262(10):4429–32. [PubMed] [Google Scholar]
- 73.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58(4):467–86. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 74.Fasbender A, Zabner J, Chillon M, Moninger TO, Puga AP, Davidson BL, et al. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J Biol Chem. 1997;272(10):6479–89. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 75.Ward CM, Read ML, Seymour LW. Systemic circulation of poly(L-lysine)/DNA vectors is influenced by polycation molecular weight and type of DNA: differential circulation in mice and rats and the implications for human gene therapy. Blood. 2001;97(8):2221–9. doi: 10.1182/blood.v97.8.2221. [DOI] [PubMed] [Google Scholar]
- 76.Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1(1):39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 77.Park JW, Mok H, Park TG. Physical adsorption of PEG grafted and blocked poly-L-lysine copolymers on adenovirus surface for enhanced gene transduction. J Control Release. 2010;142(2):238–44. doi: 10.1016/j.jconrel.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 78.Chillon M, Lee JH, Fasbender A, Welsh MJ. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 1998;5(7):995–1002. doi: 10.1038/sj.gt.3300665. [DOI] [PubMed] [Google Scholar]
- 79.Singh R, Tian B, Kostarelos K. Artificial envelopment of nonenveloped viruses: enhancing adenovirus tumor targeting in vivo. Faseb J. 2008;22(9):3389–402. doi: 10.1096/fj.08-103275. [DOI] [PubMed] [Google Scholar]
- 80.Kawamata Y, Nagayama Y, Nakao K, Mizuguchi H, Hayakawa T, Sato T, et al. Receptor-independent augmentation of adenovirus-mediated gene transfer with chitosan in vitro. Biomaterials. 2002;23(23):4573–9. doi: 10.1016/s0142-9612(02)00203-x. [DOI] [PubMed] [Google Scholar]
- 81.Park Y, Kang E, Kwon OJ, Hwang T, Park H, Lee JM, et al. Ionically crosslinked Ad/chitosan nanocomplexes processed by electrospinning for targeted cancer gene therapy. J Control Release. 2010;148:75. doi: 10.1016/j.jconrel.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 82.Chollet P, Favrot MC, Hurbin A, Coll JL. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J Gene Med. 2002;4(1):84–91. doi: 10.1002/jgm.237. [DOI] [PubMed] [Google Scholar]
- 83.Boeckle S, Wagner E. Optimizing targeted gene delivery: chemical modification of viral vectors and synthesis of artificial virus vector systems. Aaps J. 2006;8(4):E731–42. doi: 10.1208/aapsj080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278(45):44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 85.Ou M, Wang XL, Xu R, Chang CW, Bull DA, Kim SW. Novel biodegradable poly(disulfide amine)s for gene delivery with high efficiency and low cytotoxicity. Bioconjug Chem. 2008;19(3):626–33. doi: 10.1021/bc700397x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jere D, Arote R, Jiang HL, Kim YK, Cho MH, Cho CS. Bioreducible polymers for efficient gene and siRNA delivery. Biomed Mater. 2009;4(2):025020. doi: 10.1088/1748-6041/4/2/025020. [DOI] [PubMed] [Google Scholar]
- 87.Yu H, Russ V, Wagner E. Influence of the molecular weight of bioreducible oligoethylenimine conjugates on the polyplex transfection properties. Aaps J. 2009;11(3):445–55. doi: 10.1208/s12248-009-9122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim TI, Kim SW. Bioreducible polymers for gene delivery. React Funct Polym. 2011;71(3):344–349. doi: 10.1016/j.reactfunctpolym.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim TI, Ou M, Lee M, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30(4):658–64. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim PH, Kim TI, Yockman JW, Kim SW, Yun CO. The effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapy. Biomaterials. 2010;31(7):1865–74. doi: 10.1016/j.biomaterials.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 91.Kim PH, Kim J, Kim TI, Nam HY, Yockman JW, Kim M, et al. Bioreducible polymer-conjugated oncolytic adenovirus for hepatoma-specific therapy via systemic administration. Biomaterials. doi: 10.1016/j.biomaterials.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 92.Kim J, Nam HY, Kim TI, Kim PH, Ryu J, Yun CO, et al. Active targeting of RGD-conjugated bioreducible polymer for delivery of oncolytic adenovirus expressing shRNA against IL-8 mRNA. Biomaterials. 2011;32(22):5158–66. doi: 10.1016/j.biomaterials.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aggarwal N, HogenEsch H, Guo P, North A, Suckow M, Mittal SK. Biodegradable alginate microspheres as a delivery system for naked DNA. Can J Vet Res. 1999;63(2):148–52. [PMC free article] [PubMed] [Google Scholar]
- 94.Bowersock TL, HogenEsch H, Suckow M, Guimond P, Martin S, Borie D, et al. Oral vaccination of animals with antigens encapsulated in alginate microspheres. Vaccine. 1999;17(13-14):1804–11. doi: 10.1016/s0264-410x(98)00437-x. [DOI] [PubMed] [Google Scholar]
- 95.Anderson MG, Miller JD. Therapeutic effects of leuprorelin microspheres on endometriosis and uterine leiomyomata. Adv Drug Deliv Rev. 1997;28(1):139–155. [PubMed] [Google Scholar]
- 96.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 97.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6(2):215–26. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sailaja G, HogenEsch H, North A, Hays J, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9(24):1722–9. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim PH, Kang E, Choi JW, Yun TJ, Park HK, Kim JH, et al. Sustained delivery of oncolytic adenovirus in alginate gel for local tumor virotheraphy. J Control Release. 2010;148(1):e100–1. doi: 10.1016/j.jconrel.2010.07.074. [DOI] [PubMed] [Google Scholar]
- 100.Mok H, Park JW, Park TG. Microencapsulation of PEGylated adenovirus within PLGA microspheres for enhanced stability and gene transfection efficiency. Pharm Res. 2007;24(12):2263–9. doi: 10.1007/s11095-007-9441-y. [DOI] [PubMed] [Google Scholar]
- 101.Di Paolo NC, Shayakhmetov DM. Adenovirus de-targeting from the liver. Curr Opin Mol Ther. 2009;11(5):523–31. [PubMed] [Google Scholar]
- 102.Zaiss AK, Machado HB, Herschman HR. The influence of innate and pre-existing immunity on adenovirus therapy. J Cell Biochem. 2009;108(4):778–90. doi: 10.1002/jcb.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eto Y, Yoshioka Y, Mukai Y, Okada N, Nakagawa S. Development of PEGylated adenovirus vector with targeting ligand. Int J Pharm. 2008;354(1-2):3–8. doi: 10.1016/j.ijpharm.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 104.Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochem Soc Trans. 2004;32(Pt3):397–402. doi: 10.1042/BST0320397. [DOI] [PubMed] [Google Scholar]
- 105.Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat Med. 2002;8(7):751–5. doi: 10.1038/nm720. [DOI] [PubMed] [Google Scholar]
- 106.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 107.Paleolog EM. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002;4 3:S81–90. doi: 10.1186/ar575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264(5158):569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 109.Luo J, Paranya G, Bischoff J. Noninflammatory expression of E-selectin is regulated by cell growth. Blood. 1999;93(11):3785–91. [PubMed] [Google Scholar]
- 110.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60(2):203–12. [PubMed] [Google Scholar]
- 111.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology (N Y) 1995;13(3):265–70. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 112.Suh W, Han SO, Yu L, Kim SW. An angiogenic, endothelial-cell-targeted polymeric gene carrier. Mol Ther. 2002;6(5):664–72. [PubMed] [Google Scholar]
- 113.Kim WJ, Yockman JW, Lee M, Jeong JH, Kim YH, Kim SW. Soluble Flt-1 gene delivery using PEI-g-PEG-RGD conjugate for anti-angiogenesis. J Control Release. 2005;106(1-2):224–34. doi: 10.1016/j.jconrel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 114.Eto Y, Gao JQ, Sekiguchi F, Kurachi S, Katayama K, Maeda M, et al. PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J Gene Med. 2005;7(5):604–12. doi: 10.1002/jgm.699. [DOI] [PubMed] [Google Scholar]
- 115.Maeda M, Kida S, Hojo K, Eto Y, Gaob JQ, Kurachi S, et al. Design and synthesis of a peptide-PEG transporter tool for carrying adenovirus vector into cells. Bioorg Med Chem Lett. 2005;15(3):621–4. doi: 10.1016/j.bmcl.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 116.Ogawara K, Rots MG, Kok RJ, Moorlag HE, Van Loenen AM, Meijer DK, et al. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum Gene Ther. 2004;15(5):433–43. doi: 10.1089/10430340460745766. [DOI] [PubMed] [Google Scholar]
- 117.Ogawara K, Kuldo JM, Oosterhuis K, Kroesen BJ, Rots MG, Trautwein C, et al. Functional inhibition of NF-kappaB signal transduction in alphavbeta3 integrin expressing endothelial cells by using RGD-PEG-modified adenovirus with a mutant IkappaB gene. Arthritis Res Ther. 2006;8(1):R32. doi: 10.1186/ar1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Niu G, Xiong Z, Cheng Z, Cai W, Gambhir SS, Xing L, et al. In vivo bioluminescence tumor imaging of RGD peptide-modified adenoviral vector encoding firefly luciferase reporter gene. Mol Imaging Biol. 2007;9(3):126–34. doi: 10.1007/s11307-007-0079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stevenson M, Hale AB, Hale SJ, Green NK, Black G, Fisher KD, et al. Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via alpha6-integrins. Cancer Gene Ther. 2007;14(4):335–45. doi: 10.1038/sj.cgt.7701022. [DOI] [PubMed] [Google Scholar]
- 120.Parker AL, Fisher KD, Oupicky D, Read ML, Nicklin SA, Baker AH, et al. Enhanced gene transfer activity of peptide-targeted gene-delivery vectors. J Drug Target. 2005;13(1):39–51. doi: 10.1080/10611860400020449. [DOI] [PubMed] [Google Scholar]
- 121.Yao X, Yoshioka Y, Morishige T, Eto Y, Narimatsu S, Kawai Y, et al. Tumor Vascular Targeted Delivery of Polymer-conjugated Adenovirus Vector for Cancer Gene Therapy. Mol Ther. 2011;19(9):1619–25. doi: 10.1038/mt.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gotoh N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci. 2008;99(7):1319–25. doi: 10.1111/j.1349-7006.2008.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Black PC, Agarwal PK, Dinney CP. Targeted therapies in bladder cancer--an update. Urol Oncol. 2007;25(5):433–8. doi: 10.1016/j.urolonc.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 124.Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr Relat Cancer. 2001;8(1):3–9. doi: 10.1677/erc.0.0080003. [DOI] [PubMed] [Google Scholar]
- 125.Alper O, Bergmann-Leitner ES, Bennett TA, Hacker NF, Stromberg K, Stetler-Stevenson WG. Epidermal growth factor receptor signaling and the invasive phenotype of ovarian carcinoma cells. J Natl Cancer Inst. 2001;93(18):1375–84. doi: 10.1093/jnci/93.18.1375. [DOI] [PubMed] [Google Scholar]
- 126.Bonsted A, Engesaeter BO, Hogset A, Maelandsmo GM, Prasmickaite L, D'Oliveira C, et al. Photochemically enhanced transduction of polymer-complexed adenovirus targeted to the epidermal growth factor receptor. J Gene Med. 2006;8(3):286–97. doi: 10.1002/jgm.853. [DOI] [PubMed] [Google Scholar]
- 127.Morrison J, Briggs SS, Green N, Fisher K, Subr V, Ulbrich K, et al. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol Ther. 2008;16(2):244–51. doi: 10.1038/sj.mt.6300363. [DOI] [PubMed] [Google Scholar]
- 128.Park JW, Mok H, Park TG. Epidermal growth factor (EGF) receptor targeted delivery of PEGylated adenovirus. Biochem Biophys Res Commun. 2008;366(3):769–74. doi: 10.1016/j.bbrc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 129.Bonsted A, Engesaeter BO, Hogset A, Maelandsmo GM, Prasmickaite L, Kaalhus O, et al. Transgene expression is increased by photochemically mediated transduction of polycation-complexed adenoviruses. Gene Ther. 2004;11(2):152–60. doi: 10.1038/sj.gt.3302166. [DOI] [PubMed] [Google Scholar]
- 130.Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K, Moan J, et al. Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999;59(6):1180–3. [PubMed] [Google Scholar]
- 131.Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9(5):639–51. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lanciotti J, Song A, Doukas J, Sosnowski B, Pierce G, Gregory R, et al. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol Ther. 2003;8(1):99–107. doi: 10.1016/s1525-0016(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 133.Green NK, Morrison J, Hale S, Briggs SS, Stevenson M, Subr V, et al. Retargeting polymer-coated adenovirus to the FGF receptor allows productive infection and mediates efficacy in a peritoneal model of human ovarian cancer. J Gene Med. 2008;10(3):280–9. doi: 10.1002/jgm.1121. [DOI] [PubMed] [Google Scholar]
- 134.Rancourt C, Rogers BE, Sosnowski BA, Wang M, Piche A, Pierce GF, et al. Basic fibroblast growth factor enhancement of adenovirus-mediated delivery of the herpes simplex virus thymidine kinase gene results in augmented therapeutic benefit in a murine model of ovarian cancer. Clin Cancer Res. 1998;4(10):2455–61. [PubMed] [Google Scholar]
- 135.Roghani M, Moscatelli D. Basic fibroblast growth factor is internalized through both receptor-mediated and heparan sulfate-mediated mechanisms. J Biol Chem. 1992;267(31):22156–62. [PubMed] [Google Scholar]
- 136.Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63(8):803–43. doi: 10.2165/00003495-200363080-00005. [DOI] [PubMed] [Google Scholar]
- 137.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. First-line Herceptin monotherapy in metastatic breast cancer. Oncology. 2001;61 2:37–42. doi: 10.1159/000055400. [DOI] [PubMed] [Google Scholar]
- 138.Vecchione L, Orditura M, Ciardiello F, De Vita F. Novel investigational drugs for gastric cancer. Expert Opin Investig Drugs. 2009;18(7):945–55. doi: 10.1517/13543780902969455. [DOI] [PubMed] [Google Scholar]
- 139.Bange J, Zwick E, Ullrich A. Molecular targets for breast cancer therapy and prevention. Nat Med. 2001;7(5):548–52. doi: 10.1038/87872. [DOI] [PubMed] [Google Scholar]
- 140.Santin AD, Bellone S, Roman JJ, McKenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Int J Gynaecol Obstet. 2008;102(2):128–31. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 141.Jung Y, Park HJ, Kim PH, Lee J, Hyung W, Yang J, et al. Retargeting of adenoviral gene delivery via Herceptin-PEG-adenovirus conjugates to breast cancer cells. J Control Release. 2007;123(2):164–71. doi: 10.1016/j.jconrel.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 142.Kim PH, Sohn JH, Choi JW, Jung Y, Kim SW, Haam S, et al. Active targeting and safety profile of PEG-modified adenovirus conjugated with herceptin. Biomaterials. 2011;32(9):2314–26. doi: 10.1016/j.biomaterials.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 143.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–71. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 144.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1(11):1311–8. [PubMed] [Google Scholar]
- 145.del Carmen MG, Rizvi I, Chang Y, Moor AC, Oliva E, Sherwood M, et al. Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. J Natl Cancer Inst. 2005;97(20):1516–24. doi: 10.1093/jnci/dji314. [DOI] [PubMed] [Google Scholar]
- 146.Morrison J, Briggs SS, Green NK, Thoma C, Fisher KD, Kehoe S, et al. Cetuximab retargeting of adenovirus via the epidermal growth factor receptor for treatment of intraperitoneal ovarian cancer. Hum Gene Ther. 2009;20(3):239–51. doi: 10.1089/hum.2008.167. [DOI] [PubMed] [Google Scholar]
- 147.Sato H, Usuda N, Kuroda M, Hashimoto S, Maruta M, Maeda K. Significance of serum concentrations of E-selectin and CA19-9 in the prognosis of colorectal cancer. Jpn J Clin Oncol. 40(11):1073–80. doi: 10.1093/jjco/hyq095. [DOI] [PubMed] [Google Scholar]
- 148.Dabelsteen E. Cell surface carbohydrates as prognostic markers in human carcinomas. J Pathol. 1996;179(4):358–69. doi: 10.1002/(SICI)1096-9896(199608)179:4<358::AID-PATH564>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 149.Kannagi R. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj J. 1997;14(5):577–84. doi: 10.1023/a:1018532409041. [DOI] [PubMed] [Google Scholar]
- 150.Bachtarzi H, Stevenson M, Ubr V, Seymour LW, Fisher KD. E-selectin is a viable route of infection for polymer-coated adenovirus retargeting in TNF-?-activated human umbilical vein endothelial cells. J Drug Target. 2011 doi: 10.3109/1061186X.2010.547585. [DOI] [PubMed] [Google Scholar]
- 151.Willemsen RA, Pechar M, Carlisle RC, Schooten E, Pola R, Thompson AJ, et al. Multi-component polymeric system for tumour cell-specific gene delivery using a universal bungarotoxin linker. Pharm Res. 2010;27(11):2274–82. doi: 10.1007/s11095-010-0088-8. [DOI] [PubMed] [Google Scholar]
- 152.Meshkin B, Blum K. Folate nutrigenetics: a convergence of dietary folate metabolism, folic acid supplementation, and folate antagonist pharmacogenetics. Drug Metab Lett. 2007;1(1):55–60. doi: 10.2174/187231207779814319. [DOI] [PubMed] [Google Scholar]
- 153.Sirotnak FM. Obligate genetic expression in tumor cells of a fetal membrane property mediating “folate” transport: biological significance and implications for improved therapy of human cancer. Cancer Res. 1985;45(9):3992–4000. [PubMed] [Google Scholar]
- 154.Glynn SA, Albanes D. Folate and cancer: a review of the literature. Nutr Cancer. 1994;22(2):101–19. doi: 10.1080/01635589409514336. [DOI] [PubMed] [Google Scholar]
- 155.Schulz WA. DNA methylation in urological malignancies (review) Int J Oncol. 1998;13(1):151–67. [PubMed] [Google Scholar]
- 156.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994;73(9):2432–43. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 157.Zhao XB, Lee RJ. Tumor-selective targeted delivery of genes and antisense oligodeoxyribonucleotides via the folate receptor. Adv Drug Deliv Rev. 2004;56(8):1193–204. doi: 10.1016/j.addr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 158.Konda SD, Aref M, Brechbiel M, Wiener EC. Development of a tumor-targeting MR contrast agent using the high-affinity folate receptor: work in progress. Invest Radiol. 2000;35(1):50–7. doi: 10.1097/00004424-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 159.Giaginis C, Zarros A, Alexandrou P, Klijanienko J, Delladetsima I, Theocharis S. Evaluation of coxsackievirus and adenovirus receptor expression in human benign and malignant thyroid lesions. Apmis. 2010;118(3):210–21. doi: 10.1111/j.1600-0463.2009.02582.x. [DOI] [PubMed] [Google Scholar]
- 160.Glasgow JN, Everts M, Curiel DT. Transductional targeting of adenovirus vectors for gene therapy. Cancer Gene Ther. 2006;13(9):830–44. doi: 10.1038/sj.cgt.7700928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nicklin SA, Wu E, Nemerow GR, Baker AH. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol Ther. 2005;12(3):384–93. doi: 10.1016/j.ymthe.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 162.Han J, Zhao D, Zhong Z, Zhang Z, Gong T, Sun X. Combination of adenovirus and cross-linked low molecular weight PEI improves efficiency of gene transduction. Nanotechnology. 2010;21(10):105106. doi: 10.1088/0957-4484/21/10/105106. [DOI] [PubMed] [Google Scholar]
- 163.Oh IK, Mok H, Park TG. Folate immobilized and PEGylated adenovirus for retargeting to tumor cells. Bioconjug Chem. 2006;17(3):721–7. doi: 10.1021/bc060030c. [DOI] [PubMed] [Google Scholar]


