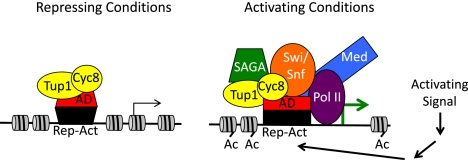
Figure 1.
Model for Tup1–Cyc8 masking of activation domains. In repressing conditions, the complex of a Tup1 tetramer with a single Cyc8 protein associates with the activation domain (AD) of the repressor–activator (Rep–Act), preventing association of SAGA, Swi/Snf, and Mediator coactivators. The absence of recruited chromatin remodelers and HATs results in a “default” state of relatively deacetylated histones and compact nucleosome structure. An environmental signal (activating condition) initiates a cascade of events leading to modification of the repressor–activator and possibly a conformational change that alters its interaction with Tup1–Cyc8 to allow recruitment of SAGA, Swi/Snf, and Mediator and subsequent acetylation of histones and disruption of nucleosomes. Tup1–Cyc8 remains at the promoter and may participate in coactivator recruitment.