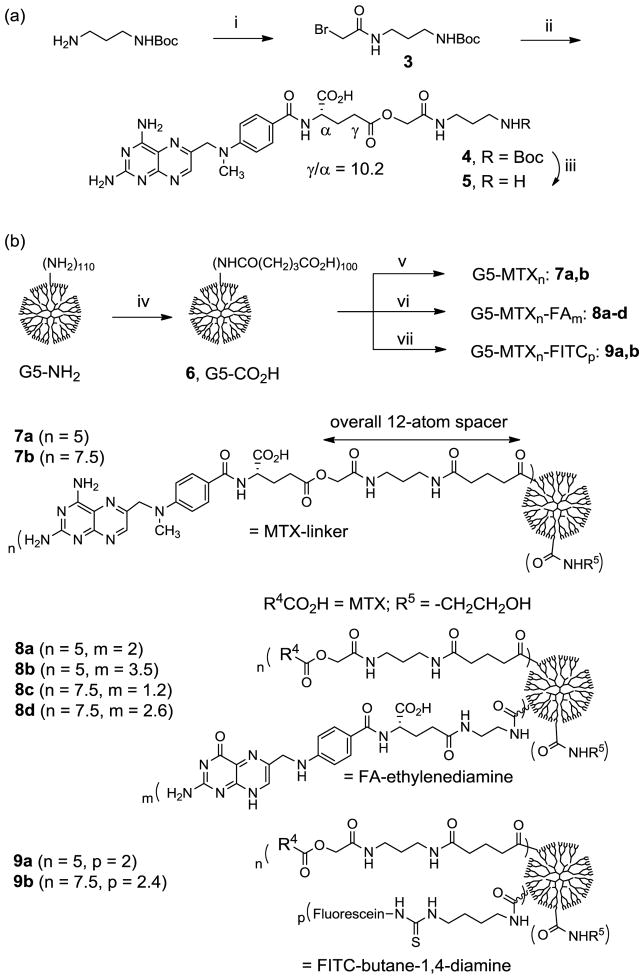
Scheme 2.
(a) Synthesis of a new methotrexate-linker construct. Reagents and conditions: i) bromoacetyl chloride, DIPEA, CHCl3, 0°C to rt, 24 h, 64%; ii) methotrexate hydrate, Cs2CO3, K2CO3, NaI, DMF, rt, 48 h, 22%; iii) TFA, CH2Cl2, rt, 15 min; (b) Synthesis of generation 5 (G5) PAMAM dendrimer-based nanoconjugates 7a,b, 8a-d, and 9a,b with variable amounts of methotrexate attached singly or in combination with folic acid molecules. iv) EDC, NHS, DMF, rt, 36 h; v) 5, Et3N, DMF, rt, 24h; then ethanolamine; vi) 5, FA-CONH(CH2)2NH2, Et3N, DMF, rt, 24h; ethanolamine; vii) 5, fluorescein-NHC(=S)NH(CH2)4NH2, Et3N, DMF, rt, 24h; ethanolamine.