Figure 2. CYLD in cell signaling.
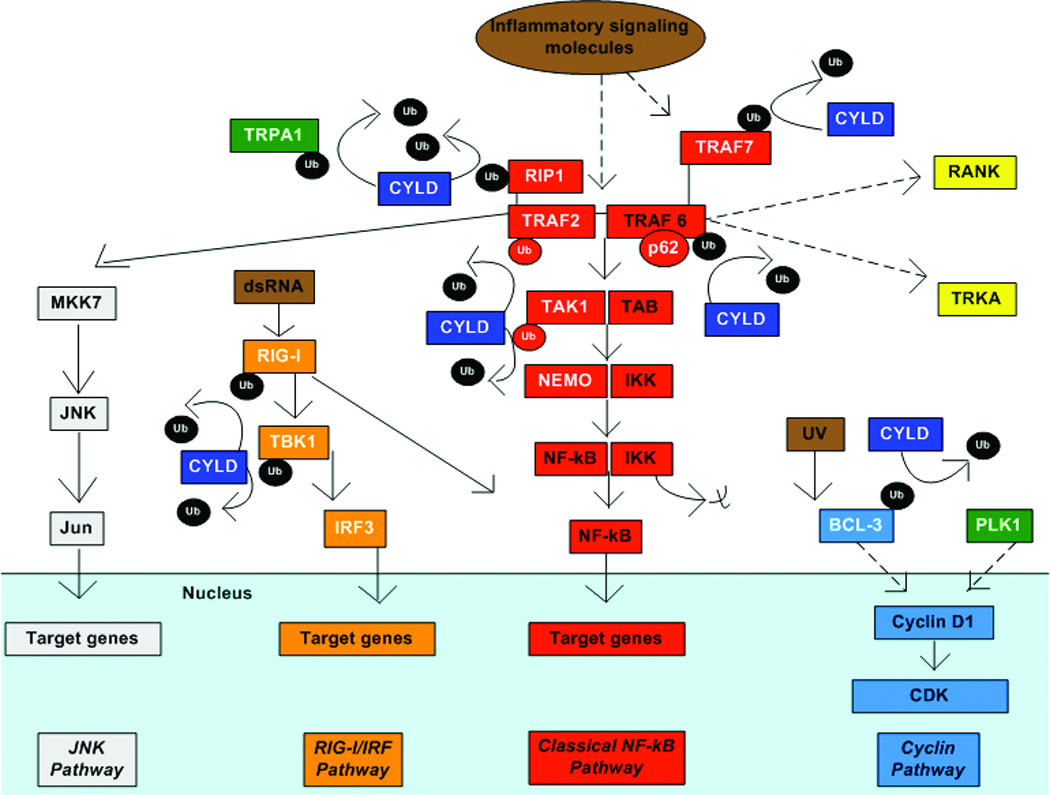
Model of how CYLD regulates different signaling pathways regulating survival, proliferation and inflammation in different cell types through deubiquitination. Dotted arrows denote multistep or incomplete pathways. CYLD and its known binding partners are in white font. Targets activated by CYLD are in green. Key targets for CYLD include removal of K63-linked Ub from TRAF2 and TRAF6, proteins that form a complex for NF-kB signaling. CYLD also deubiquitinates RIP1, a protein that associates with TRAF2. Deubiquitination of TRAF2 inhibits MKK7 activation and downstream JNK signaling. Deubiquitination of TRAF2, TRAF6, TRAF7 and TAK1 inhibit downstream dissociation of IKK from NF-kB and NF-KB signaling. In addition, deubiquitination of TRAF6 also inhibits downstream activation of RANK signaling and internalization of the TRKA receptor. CYLD interaction with TRAF6 requires the adaptor protein p62. In response to dsRNA, RIG-I is activated. CYLD DUB of RIG-I and TBK1 inhibits downstream activation of IRF3 signaling. RIG-I is a known activator of NF-kB as well. CYLD deubiquitination of BCL3 prevents its translocation from the cytoplasm to the nucleus in response to UV light. Nuclear BCL-3 activates cyclinD1 and promotes cell proliferation. CYLD also activates cyclinD1, possibly through PLK1. CYLD associates with the calcium channel, TRPA1, and maintains its activation by removing ubiquitin.
