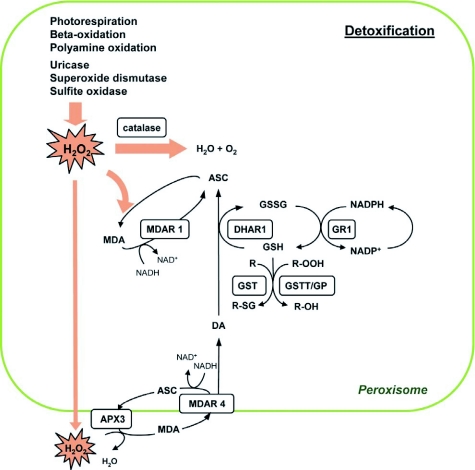
Figure 7.
Model for detoxification reactions in plant peroxisomes.
H2O2 is a potent ROS generated as a by-product of various metabolic reactions occurring within the peroxisome. It has the potential to inflict severe damage to enzymes and membrane lipids, thus necessitating its rapid detoxification. H2O2 is scavenged either by the catalases (CAT1, -2, and -3) in the matrix or through the activity of the ascorbate (ASC)-glutathione (GSH) cycle. In the latter case, H2O2 is reduced to H2O by ascorbate peroxidase (APX), producing monodehydroascorbate (MDA). MDA can either be reduced to ASC by monodehydroascorbate reductase (MDAR) with the simultaneous oxidation of NADH to NAD+ or, alternatively, MDA undergoes disproportionation to yield ASC and dehydroascorbate (DA). MDAR isoforms are associated both with the peroxisomal matrix (MDAR1) and the peroxisome membrane (MDAR4). Dehydroascorbate reductase (DHAR) acts on DA, utilizing reduced glutathione (GSH) to reduce DA to ASC; GSH is oxidized to GSSG in this reaction. Glutathione reductase (GR) regenerates GSH by using the reduced cofactor NADPH. Peroxisomal isocitrate dehydrogenase and 6-phosphogluconate dehydrogenase probably catalyze the reduction of NADP+ to NADPH, regenerating the reduced cofactor for the operation of GR (not shown). Three members of the theta family of glutathione S-transferases (GSTT) function mainly as glutathione peroxidases (GP), reducing fatty acid hydroperoxides (R-OOH) and H2O2 at the expense of GSH, although they also show limited transferase activity in conjugating GSH to metabolite (R).